Request More Information
Data Integrity Solutions

From the synthesis of the first drug candidate through to QA and lot release, the quality, integrity, and accessibility of your data is key to the success of delivering new therapeutics safely and effectively to the world. With the strength of Shimadzu as a multi-instrument solution provider, we have built a software ecosystem that allows users to imagine single-point workflows that span every aspect of analytical measurement and analysis. Imagine the ability to integrate data from chromatography, molecular spectroscopy, elemental spectroscopy, as well as gas and liquid phase mass spectrometry within a single report, from a single software platform. LabSolutions is a scalable solution for your laboratory data, from both Shimadzu instruments and non-Shimadzu data sources, that offers cGMP and FDA compliance features to ensure the integrity and quality of your results. We have assisted our partners to create globally integrated instrument control and reporting solutions that meet the regulatory demands and increase user efficiency. Whether your task requires enterprise-level deployment of multiple instruments, or integrating a stand-alone solution into an existing informatics structure, Shimadzu provides solutions that give you confidence in the integrity of your data.
Featured Applications
Data Integrity Compliance in the Analytical Laboratory
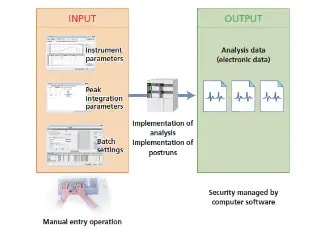
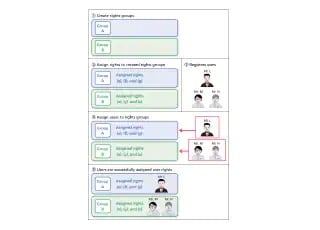
Data integrity means the completeness of data. It expresses an assurance that all the data is included and that there are no inconsistencies. LabSolutions DB/CS software enables compliance with data integrity requirements by providing a total system for managing analytical instruments and test information in a single location. It not only improves the efficiency of testing processes, but also prevents data tampering.
Data Integrity Compliance: An Innovative Solution for Molecular Spectroscopy
Regulatory authorities for analytical instruments are not only interested in chromatography systems, such as liquid chromatographs and gas chromatographs, but are also turning their interest into spectroscopy systems such as UV and IR systems. This report describes an innovative solution for ensuring data integrity for such spectroscopy systems.
Data Integrity with LabSolutions White Paper
In this white paper, Shimadzu outline the controls that are needed to address data integrity in the laboratory, provide an overview of the procedural and behavioural issues that must be addressed and explain how Shimadzu fulfil their responsibilities for technical compliance with regulatory requirements for data integrity through the use of their LabSolutions CS analysis data management system
Data Integrity Compliance with LabSolutions Report Set
LabSolutions DB/CS version 6.50 includes a new Report Set function that enables the visibility of software operations. Not only can it help ensure the reliability of the analysis data required by analysis laboratories, but, it can also decrease the amount of time needed to check analysis results to a half or third of that previously required.
Practical Procedures for Compliance with Data Integrity Requirements in Analytical Laboratories
This report describes practical procedures for ensuring the data integrity for analytical instruments.
News / Events
-
AAPS Pharm Sci 360 2024
October 21-23
Salt Palace Convention Center
Salt Lake City, Utah
Booth #2107
-
ASMS (American Society for Mass Spectrometry) 2024
June 2-6
Anaheim Convention Center
Anaheim, California
-
ASMS (American Society for Mass Spectrometry) 2024
June 2-6
Anaheim Convention Center
Anaheim, California