Request More Information
QA/QC Manufacturing

The importance of reliable, rigorous quality analysis and quality control in pharmaceutical manufacture and distribution cannot be overstated. Consumers trust your products to safely and effectively deliver medicines that improve and sustain life. In order to provide this assurance across a wide variety of therapeutic modalities and drug delivery mechanisms, you need a partner that understands how to build a solution that unifies instrument results, data integrity, and regulatory compliance. Shimadzu has a broad range of analytical instruments to meet your QA/QC needs, and bring together data management and reporting capabilities that goes far beyond the chromatography data system to a laboratory data system.
Whether your tasks involve routine potency and impurities analysis by USP monograph, or testing the push-out strength of blister pack OTC tablets, Shimadzu has the instruments, service and support to help you maintain up time and avoid Out of Specification results before they happen.
Featured Topics
Particle Size Analysis for Pharmaceuticals

Powders are often used as active pharmaceutical ingredients (APIs) and intermediates of pharmaceuticals. The particle size of APIs and intermediates affects the efficacy of medicines since particle size has an impact on the absorption rate in the gastrointestinal tract after the medicine is taken. It is also important to measure the particle characteristics of raw materials to ensure stable quality production of pharmaceuticals.
USP Application Compendium

Learn more about USP monograph and other applications for FTIR, GC, Residual Solvents per USP <467>, HPLC including modernization of methods per USP <621>, Physical Testing per USP <915> and <1912>, TOC per USP <643> and <661.1-2>.
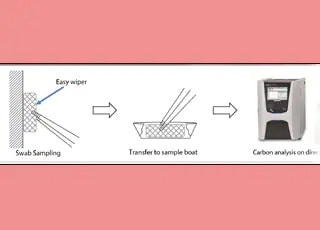
Cleaning Validation
Particle Size Monitoring
Physical Testing
Raw Materials Testing

Thermal Analysis
Related Products
News / Events
-
AAPS Pharm Sci 360 2024
October 21-23
Salt Palace Convention Center
Salt Lake City, Utah
Booth #2107
-
ASMS (American Society for Mass Spectrometry) 2024
June 2-6
Anaheim Convention Center
Anaheim, California
-
Shimadzu Scientific Instruments Opens R&D Center
These forward-focused R&D centers will enable us to closely collaborate with customers and stakeholders to develop products that meet tomorrow’s analytical laboratory needs and swiftly deliver these solutions to the marketplace.