This function gathers operational information (operations and settings that require human intervention) distributed within the software, and collects it in a single report.
Improving the Data Integrity of Spectrometers
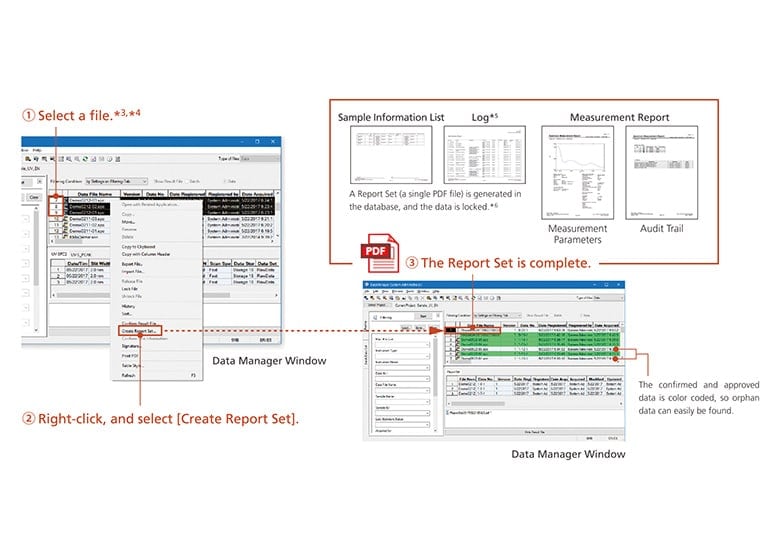
Data Integrity and Report Set
Data integrity refers to the completeness of data, with nothing missing or inconsistent. In other words, not only the data itself but also the metadata (the results of work that require human intervention, such as specifying conditions and analyzing analysis data) must be presented in a visible form, and verified together with the data. This is achieved by the Report Set.
Why Data Integrity Is Important?
The following warning letters have been issued by the FDA, demanding swift response regarding analytical instruments that do not support data integrity.
| Warning Letter: 320-18-55 Issue Date: May 23, 2018 You did not have procedures for reviewing audit trails or electronic data for the Fourier-transform infrared spectroscopy or ultraviolet systems. |
| Warning Letter: 320-17-25 Issue Date: February 24, 2017 Our investigator observed that your laboratory systems lacked controls to prevent your staff from altering or deleting electronic data. Analysts manipulated and deleted audit trails. You lacked adequate controls for all HPLC, gas chromatography, and ultra-violet systems. |
| Warning Letter: 320-17-01 lssue Date: October 13, 2016 In response, to this letter, provide details of your retrospective review of HPLC and other laboratory data, such as Fourier transform infrared spectroscopy, gas chromatography, UV spectrophotometry, and (b)(4) analyzer data. |
| Warning Letter: 320-15-09 lssue Date: April 6, 2015 You lacked controls to prevent the unauthorized manipulation of your laboratory’s electronic raw data. Specifically, your infrared (IR) spectrometer did not have access controls to prevent deletion or alteration of raw data. |