What are “short path” cuvettes and why are they used? (sample too concentrated)
In the case of high-concentration samples, diluting the sample allows measurement with a 10-mm cell. There are samples, however, that cannot be diluted easily. For example, due to interaction with the solvent, diluting a sample may cause a change in the absorbance (i.e., a shift in the peak wavelengths).
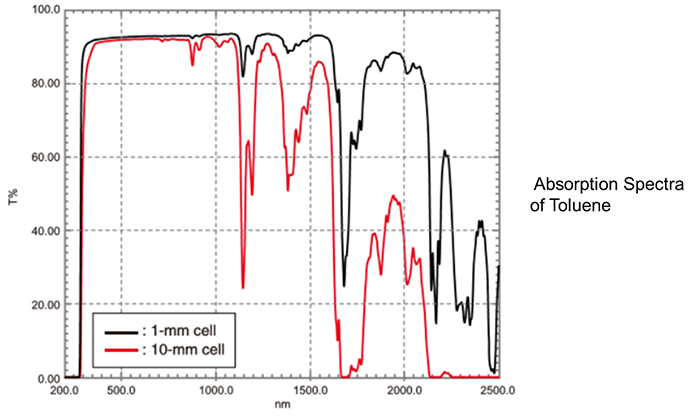
In cases where the absorbance is high, and dilution is difficult, measurement using a "short-path cell" is effective. There are short-path cells with optical path lengths of 1 mm, 2 mm, and 5 mm. The figure shows the results obtained by analyzing toluene with a 1-mm cell and a 10-mm cell. It can be seen that there is much less absorption saturation (i.e., 0 % transmittance) with the 1-mm cell than there is with the 10-mm cell.
A well-known application of measurement using a short-path cell is solution analysis in the near-infrared region. If a 10-mm cell is used for measurement in the near-infrared region, saturation often occurs due to absorption by the solvent, making it impossible to ascertain the absorption of the sample. A short-path cell is used to prevent absorption saturation due to the solvent.
With low- and high-concentration samples, the optical path length of the cell is selected in accordance with the size of the absorbance or transmittance.


