What is the output of a halogen lamp?
Similar to a normal incandescent lamp, a halogen lamp filament heats up and emits light when current flows through it. The tungsten used as the filament material evaporates at high temperatures. Consequently, the bulb containing the filament of a normal incandescent lamp is filled with an inert gas to prevent evaporation of the tungsten.
A halogen lamp contains a halide as well as the inert gas to create the halogen cycle that returns evaporated tungsten to the filament, resulting in a long lamp life. It also restricts blackening of the tube wall, due to adhering evaporated tungsten, to create a light source that remains bright over long periods. Tungsten that evaporates at high temperatures binds with halogen near the cool tube wall to form tungsten halide. The suspended tungsten halide moves inside the tube due to convection and separates into halogen and tungsten near the hot filament. The separated tungsten adheres to the filament and the halogen bonds again with evaporated tungsten. This repeated reaction is known as the tungsten cycle.
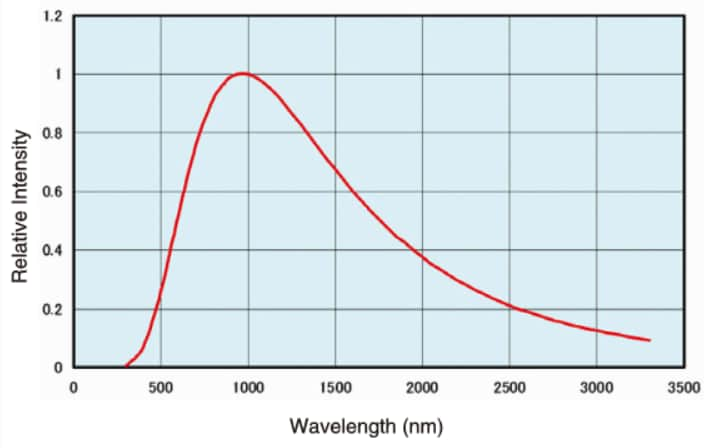
The figure here shows the light intensity distribution at 3000 K color temperature. The usable wavelength range is 350 nm to 3500 nm, but this is affected by the color temperature.
Halogen lamps are stable over time, offer a long service life (approx. 2000 hours) and are relatively inexpensive. As such, they many of the conditions required for a spectrophotometer light source.


