What is the relationship between absorption spectroscopy and sigma orbital bonding?
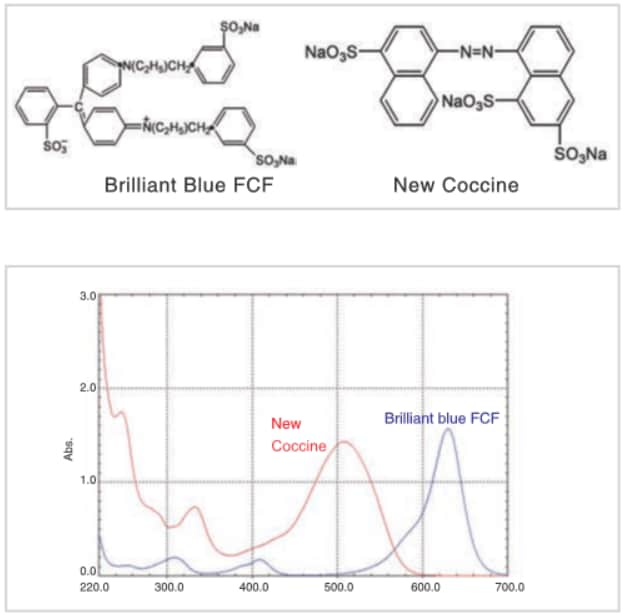
The top figure shows the structures of food dyes New Coccine (Red No. 102) and Brilliant Blue FCF (Blue No. 1) and the bottom figure shows their absorption spectra. Food dyes tend to have large conjugated systems, like those shown in figure, and therefore their peak wavelengths tend to be shifted toward the long wavelength region, with peaks appearing in the visible region (400 to 700 nm). Therefore they are recognized as colors. Incidentally, the color that we see is the color that is not absorbed by the substance (which is called the “complementary color”). As shown in the figure, New Coccine absorbs blue and green light in the range 450 to 550 nm, and so the complementary color, red, is seen by the human eye. Brilliant Blue FCF absorbs yellow light in the range 560 to 650 nm and so blue is seen by the human eye.


