Why is the wavelength of light important?
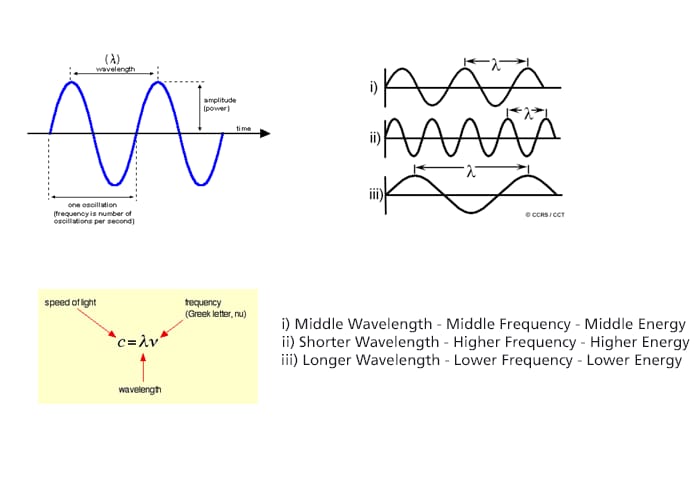
To answer this question, we need to know some important characteristics of light in general. Any wave is essentially just a way of shifting energy from one place to another, whether the fairly obvious transfer of energy in waves on the sea or in the much more difficult to imagine waves in light. With waves on water, the energy is transferred by the bulk movement of water molecules; however, a particular water molecule doesn’t travel all the way across the Atlantic, or even all the way across a pond. Depending on the depth of the water, waves follow a roughly circular path from the point of origin. As they move up to the top of the circle, the wave builds to a crest; as they move down again, you get a trough. The energy is transferred by relatively small local movements in the environment. With water waves it is fairly easy to draw diagrams to show this happening with real molecules. With light it is more difficult. The energy in light travels because of local fluctuating changes in electrical and magnetic fields; hence “electromagnetic” radiation. (Upper Left)
If you draw a beam of light in the form of a wave, without worrying too much about what exactly is causing the wave, the distance between two crests is called the wavelength of the light. It could equally well be the distance between two troughs or any other two identical positions on the wave. You have to picture these wave crests as moving from left to right. If you counted the number of crests passing a particular point per second, you have the frequency of the light. It is measured in what used to be called “cycles per second”, but is now called Hertz, Hz. Cycles per second and Hertz mean exactly the same thing. Light has a constant speed through a given substance. For example, it always travels at a speed of approximately 3 x 108 meters per second in a vacuum. This is actually the speed that all electromagnetic radiation travels not just visible light. There is a simple relationship between the wavelength and frequency of a particular color of light and the speed of light. (Lower Left)
This relationship means that if you increase the frequency, you must decrease the wavelength and, of course, the opposite is true. If the wavelength is longer, the frequency is lower. It is really important that you feel comfortable with the relationship between frequency and wavelength. Each particular frequency of light has a particular energy associated with it. The higher the frequency, the higher the energy of the light. Light which has wavelengths of around 380 nm to 435 nm is seen as a sequence of violet colors. Various red colors have wavelengths around 625 nm to 740 nm. Which has the highest energy? The light with the highest energy will be the one with the highest frequency, that will be the one with the smallest wavelength. (Right)


