What light wavelength regions are used?
In UV/Vis/NIR spectroscopy the ultraviolet (170 nm to 380 nm), visible (380 nm to 780 nm), and near infrared (780 nm to 3300 nm) are used. A nanometer (nm) is 10-9 meter. Most spectrophotometers are configured as either as UV/Vis instruments that cover the 190 nm to 900 nm (or 1100 nm) wavelength range or UV/Vis/NIR instruments that cover the 175 nm to 3300 nm wavelength range.
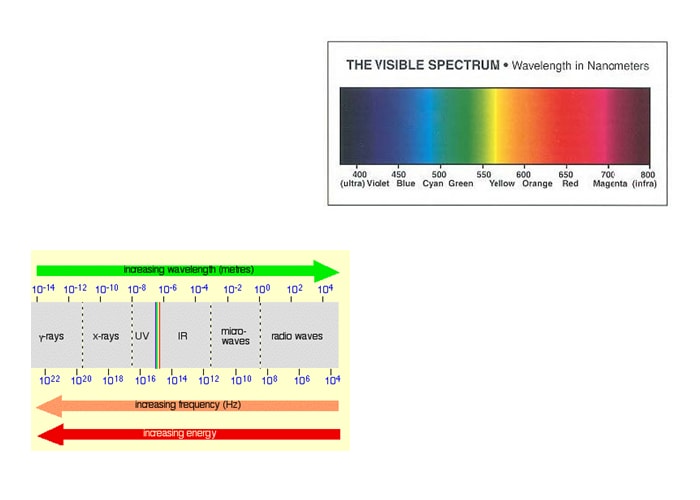
People are very familiar with the visible light region, since these are the wavelengths that the human eye is able to see. The diagram “The Visible Spectrum” above shows an approximation to the spectrum of visible light. The color order in the visible region is easy to remember using the mnemonic Roy G Biv. So, from long wavelength to short the colors are red, orange, yellow, green, blue, indigo, violet. Now we must place the visible light region into the rest of the electromagnetic spectrum. This electromagnetic spectrum doesn’t stop with the colors you can see. It is perfectly possible to have wavelengths shorter than violet light or longer than red light. The figure above shows ultraviolet and the infrared as the extremes, but this can be extended even further into x- rays and radio waves, amongst others. The diagram above shows the approximate positions of some of these on the spectrum.
Don’t worry too much about the exact boundaries between the various sorts of electromagnetic radiation, because there are no boundaries. Just as with visible light, one sort of radiation merges into the next. Just be aware of the general pattern. Also, be aware that the energy associated with the various kinds of radiation increases as the frequency increases (or wavelength decreases).


