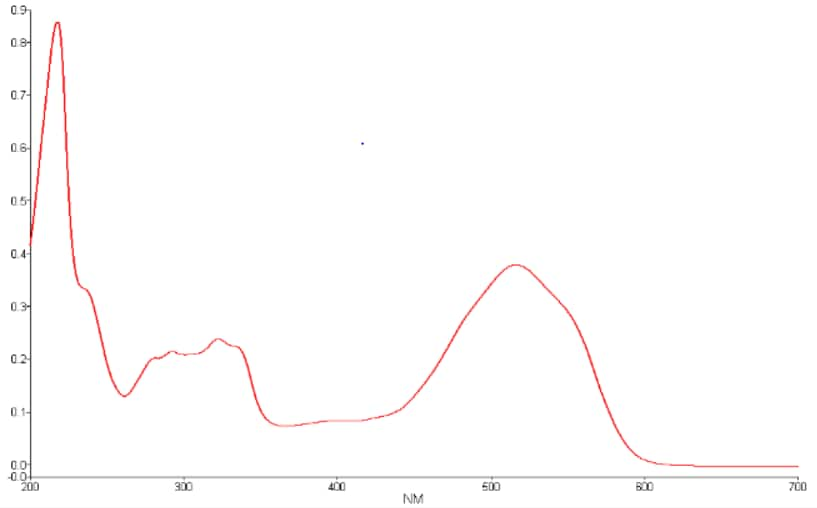
What type of information does a spectrophotometer produce?
UV/Vis spectroscopy is routinely used in analytical chemistry for the quantitative determination of different analytes, such as transition metal ions, highly conjugated organic compounds, and certain biological macromolecules. Measurement is usually carried out in solution. Solutions of transition metal ions can be colored (i.e., absorb visible light) because d electrons within the metal atoms can be excited from one electronic state to another. The color of metal ion solutions is strongly affected by the presence of other species, such as certain anions or ligands. For instance, the color of a dilute solution of copper sulfate is a very light blue; adding ammonia intensifies the color and changes the wavelength of maximum absorption (λmax). Organic compounds, especially those with a high degree of conjugation, also absorb light in the UV or visible regions of the electromagnetic spectrum. The solvents for these determinations are often water for water soluble compounds, or ethanol for organic soluble compounds. Some organic solvents may have significant UV absorption; not all solvents are suitable for use in UV spectroscopy. Ethanol absorbs very weakly at lower ultra-violet wavelengths. Solvent polarity and pH can affect the absorption spectrum of certain organic compounds.
The output of a spectrophotometer is a spectrum (usually). A spectrum (plural spectra) is a graph displaying either values for transmitted, absorbed, or reflected light (Y axis) vs. various light wavelength values (X axis).


