Simultaneous Analysis of Formic Acid and Formaldehyde
Artificial photosynthesis uses photocatalytic reactions for water splitting and carbon dioxide reduction to create hydrogen, oxygen, carbon monoxide, and formic acid. Of these reaction products, hydrogen, oxygen, and carbon monoxide escape from the sample solution as gases. However, formic acid remains in the sample together with added components, such as electron donors, and byproducts, such as formaldehyde. To measure the amount of formic acid created, it is necessary to separate it from these components. This Data Sheet highlights the pH-buffered electroconductivity method offered by the Shimadzu Organic Acid Analysis System to analyze formic acid, together with a refractive index detector for the simultaneous analysis of formaldehyde.
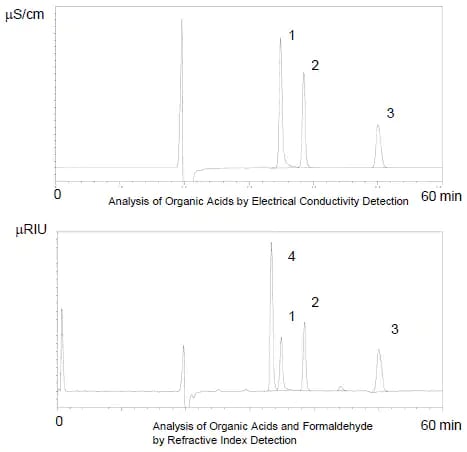
Shimadzu’s Organic Acid Analysis System pH-buffered electroconductivity method offers excellent separation and selectivity. However, as formic acid and formaldehyde overlap in the Shim-pack SCR-102H ion-exclusion column used in this system, a YMC Hydrosphere C18 reverse-phase column was added to improve separation.
In addition, as formaldehyde cannot be detected by an electrical conductivity detector, a refractive index detector was added in series.
The diagrams to the right show the analysis of several hundred ppm standard samples of each component.
Peaks:
1. Formic Acid
2. Acetic Acid
3. Propionic Acid
4. Formaldehyde
Analytical Conditions
| Columns | YMC Hydrosphere C18 (150 mmL. x 4.6 mmI.D.) Shim‐pack SCR‐102H (300 mmL. x 8.0 mmI.D.) x 2 |
| Mobile phase | 5 mmol/L Perchloric Acid |
| Flow rate | 0.6 mL/min |
| Column temp. | 40°C |
| Reaction reagent | 5 mmol/L Perchloric Acid 20 mmol/L Bis‐Tris0.1 mmol/L EDTA‐4H |
| Flow rate | 0.6 mL/min |
| Reaction temp. | 40°C |
| Detections | Electrical Conductivity, Reflective Index |
| Injection volume | 100 μL |
Simultaneous Analysis of Formic Acid and Formaldehyde
Shimadzu's unique post-column pH-buffered electroconductivity method is ideal for the selective and highly sensitive detection of organic acids. Compared to conventional methods, such as the UV short wavelength method or a simple conductivity method, this system improves quantitation reliability.


