Request More Information
Methods of Analysis

Analytical method development begins early in drug discovery and evolves throughout the drug life cycle. As a drug candidate moves through stages of development, there is a need to identify impurities whether they through formulation and stability in early stages, the inorganic contaminants that enter through drug manufacturing, or the extractables and leachables from plastics and packaging of drug products as it reaches commercialization. While the needs for a drug candidate changes through its development lifecycle, the need for sophisticated chromatography and detection techniques remain consistent.
To meet those needs, Shimadzu offers a variety of solutions that can meet the needs of analytical method development including chromatographic method development platforms, time-of-flight (TOF) and quadrupole mass spectrometry, a full suite of molecular spectroscopy equipment, and powerful software tools based on LabSolutions to prepare your lab for the demands of analytical method development. With workflows to support photo diode array peak deconvolution, impurities discovery, and LC method transfer, we give you insight to catch coeluting impurities even without separation and move methods from system to system. Add in compliance-enabled integration across multiple instrument types including balances, physical testing, LC, GC, LC-MS, GC-MS, ICP-OES, ICP-MS, FTIR, UV, Fluorometry, and TOC, to see how Shimadzu and LabSolutions can help you create efficient and robust analytical methods that move from development to QA/QC with ease.
Method Scouting Solution for UHPLC/HPLC/SFC
Method Scouting System is a method development system based on Shimadzu’s UHPLC technology. The combination with Method Scouting Solution dedicated control software achieves a fast and accurate method scouting workflow, offering excellent support for method development.
Method Development System
Method Scouting System is a method development system based on Shimadzu’s UHPLC technology. The combination with Method Scouting Solution dedicated control software achieves a fast and accurate method scouting workflow, offering excellent support for method development.
Featured Applications
Method Development Applications
High Efficiency Method Scouting for Small Molecule Drugs
The columns (stationary phase) and mobile phases used are parameters which have a large effect on retention and separation of target compounds in HPLC analysis. Conducting method scouting first to narrow the options of columns and mobile phases is an extremely effective approach for improving the efficiency of method development. However, in the process of method scouting, it is necessary to actually perform the analyses under various conditions in order to determine the optimum method for the target compounds from among the large number of possible mobile phases (buffer solution pH, salt concentration, organic solvent ratio) and columns (ODS, C8, phenyl). Moreover, considerable time, labor, and skill are also required in work such as the switching of columns and preparation/setting of the mobile phases. This article introduces the Nexera Method Scouting System and the dedicated software "Method Scouting Solution", which were developed to automate the method scouting workflow, through a example of scouting conditions for simultaneous analysis of small molecule drugs.
Efficient Method Development by automated pH Screening with LabSolutions MD
Because the pH level of mobile phase can affect the retention time of ionic compounds, determining the optimal pH level is an important part for developing LC method. The pH screening requires preparing multiple mobile phases with different pH levels. However, preparing these mobile phases manually is not only a time-consuming process but also prone to the operator errors. Automated pH screening process improves the method development efficiency and also the reliability of analysis. This article describes an example of using LabSolutions MD, a dedicated software for supporting method development, to automate pH screening by varying the mobile phase pH from 2.5 to 8.5 to evaluate the optimal pH level for separating 12 small-molecule drugs.
Efficient Method Development on Pharmaceutical Impurities Using Single Quadrupole Mass Spectrometer
Since pharmaceutical impurities require strict control to ensure safety, development of highly reliable analytical method is essential. LabSolutions MD, a new Shimadzu software for method development, supports efficient method development based on Analytical Quality by Design (AQbD). AQbD-based analytical method development consists of the phases of initial screening, optimization, and robustness evaluation. This article introduces an example of its use for the development of a robust LC method for impurities on Montelukast (a medication used in the maintenance treatment of asthma). By changing each parameter of gradient program, the resolution of Montelukast and each impurity was evaluated by visualizing through “design space.” Though it was previously difficult to accurately track each impurity with similar UV spectra using photodiode array detector (PDA), LCMS-2050 can solve this problem. Furthermore, by utilizing design space of resolution and RT of last eluting peak, it is possible to efficiently develop method that provides both excellent resolution and shorter analysis time.
Fast Analysis of Ciclesonide Inhalant by Online SFE-SFC-QTOFMS
Checking the content of active ingredients and impurities is essential for maintaining pharmaceutical quality, so establishing a quick and convenient screening method would help shorten development periods and promote practical use of pharmaceuticals. Online SFE-SFC-QTOFMS systems use a combination of supercritical fluid extraction (SFE) and supercritical fluid chromatography (SFC) for extracting components with supercritical fluid in an extraction vessel, injecting the extracted components into an SFC unit for online column separation, followed by accurate mass analysis of separated components in a quadrupole-time of flight (QTOF) mass spectrometer system. The online SFE-SFC system can also add a polar organic solvent to supercritical carbon dioxide fluid as a modifier to enable extraction and separation of components with a wide range of polarities. This article describes an example of using the online SFE-SFCQTOFMS method for screening analysis of active ingredients and impurities in the drug Alvesco, a ciclesonide inhalant used to treat bronchial asthma.
Molecular Impurities Analysis Applications
Solutions for Pharmaceutical Impurities
In this handbook, we share a variety of methodologies for different classes of impurities like elemental, organic, residual solvent and more. Methods described are in synchronization with relevant ICH guidelines and create a sound platform for scientists to initiate their quest for answers with confidence.
Contaminant Analysis of Pharmaceuticals (Tablets) Using AIRsight Infrared/Raman Microscope
In recent years, consumers have shown heightened concern about contamination of products by foreign matter, and demand for analysis to respond to this problem has also increased. Although news reports that contaminants have been discovered in some foods and pharmaceuticals have appeared from time to time, it is difficult to eradicate this problem completely, as the causes of contamination are assumed to include various processes such as contamination of raw materials at the time of purchase, contamination of the product due to deterioration of component parts of the production line, and contamination of the product by the consumer. The types of foreign matter are also diverse, including not only organic materials such as human hair, plastics, and rubber, but also oxides, metal fragments, and other inorganic substances. For these reasons, higher accuracy is required in qualitative analysis in order to identify the cause of contamination. The AIRsight Infrared/Raman microscope is a new microscope in which a Raman unit is incorporated in an infrared microscope, making it possible to carry out both Raman and infrared analysis with a single instrument, even though separate instruments had been required until now. Fig. 1 shows the appearance of the AIRsight. Since the infrared spectrum and Raman spectrum can be acquired at the same location, without moving the sample, the accuracy of qualitative analyses of micro regions is dramatically improved. Operation is also simple because both the infrared and Raman measurements can be controlled with one software program, AMsolution. This article introduces an example of measurement of a contaminant adhering to the surface of a pharmaceutical (tablet) (hereinafter, “tablet”) by micro-infrared spectroscopy and micro-Raman spectroscopy.
Simplified approach for structural elucidation and quantitation for pharmaceutical API and related impurities
Structural elucidation for pharmaceutical impurities involves multitude of analytical techniques, such as NMR, mass spectrometry, infrared spectroscopy etc. A sound decision can be made by compiling the shortlisted candidates from different tools and mapping them appropriately. Nevertheless, highly probable structure(s) can be predicted using HR-MS technique, along with prior background information. This study has been carried out for structural elucidation of paracetamol and its related impurities followed by quantitation of representative impurities using LCMS-9030 (Shimadzu Corporation, Japan), a Quadrupole Time of ight liquid chromatograph mass spectrometer. The LCMS-9030 is built using a remarkably stable TOF tube iRefTOFTM, to deliver both high resolution and accurate mass with stability; attributes essential for compound identification, confident formula assignment and quantitation.
Structural analysis of impurities in pharmaceutical ingredients using trap-free 2D-LC high-resolution accurate mass spectrometry
High performance liquid chromatography (HPLC) is the primary means for impurity testing in pharmacopeia. Solutions containing non-volatile salts are frequently used as a mobile phase because of the irreplaceable advantage to achieve better chromatographic performances. However, non-volatile mobile phases are not compatible with mass spectrometry which is commonly used for structural analysis of impurities. Trap-free two-dimensional HPLC (trap-free 2D-LC) can fill this gap by exchanging a non-volatile mobile phase with a volatile one compatible for mass spectrometry. In this study, we demonstrate how structural analysis of impurities in pharmaceutical ingredients is feasible by using trap-free 2D-LC coupled to quadrupole time-of- ight mass spectrometry (QTOF MS) without modifying the original method mobile phases.
Analysis of Impurities in Atorvastatin Using Single Quadrupole Mass Spectrometer
In addition to the API, drug substances contain trace amounts of impurities such as side reaction products, unreacted residues, and degradation products. When impurities are contained above the threshold specified in ICH-Q3, it is necessary to confirm their structure and safety. The HPLC-UV method is generally used to evaluate the purity, and if an impurity is detected, it is required to confirm its identity. In this analysis, it is a standard approach to further connect a mass detector (LC-MS) to obtain molecular weight information that helps with impurity identification. Using the LC-MS system, it is possible to further deduce the molecular structure by performing MS/MS analysis. In this report, we present an example of an analysis of a pharmaceutical product containing impurities using the LCMS2050 high-performance liquid chromatography-mass spectrometer. We will show how mass information can be automatically added to absorbance detector data for easy peak identification, and furthermore, how impurities can be analyzed and identified using in-source CID.
Improved Analytical Workflow for Phospholipids by Nexera-e and Co-Sense for Impurities
Together, the comprehensive two-dimensional Nexera-e, offering exhaustive analysis of diverse compounds, and the Co-sense series, permitting high resolution and enrichment of specific fractions, make it possible to compare the overall compound patterns in the target sample. Combination of the Nexera-e and Co-Sense series also allows precise separation of the elution fraction of interest. With this method, the two workflows consisting of the differential analysis of a sample with a complex matrix, and a detailed analysis of the compounds of those results to reveal differences, can be conducted simultaneously, quickly and conveniently. Here, we introduce an actual example using phospholipids.
Isolation and identification of Fluticasone degradation impurities by UFPLC
Fluticasone propionate, a medium-potency synthetic corticosteroid, is used topically to relieve inflammatory and prurtic symptoms of dermatoses and psoriasis, intranasally to manage symptoms of allergic and non-allergic rhinitis, and orally for the treatment of asthma. Fluticasone Proprionate is marketed under several different brand names such as Flonase®. Fluticasone propionate is also available as a combination product of Azelastine Hydrochloride and Fluticasone Propionate called Dymista™. Dymista™ is indicated in patients over 12 years old for symptomatic relief of seasonal allergic rhinitis.
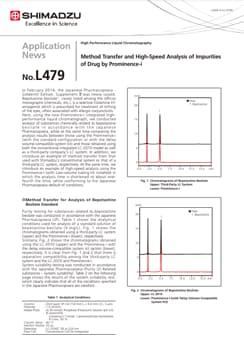
Method Transfer and High-Speed Analysis of Impurities of Drug by Prominence-i
In February 2014, the Japanese Pharmacopoeia - Sixteenth Edition, Supplement Ⅱ was newly issued. Bepotastine besilate1), newly listed among the official monographs (chemicals, etc.), is a selective histamine H1 antagonist which is prescribed for treatment of itching of the eyes, often associated with allergic conjunctivitis. Here, using the new Prominence-i integrated high performance liquid chromatograph, we conducted analysis of substances chemically related to bepotastine besilate in accordance with the Japanese Pharmacopoeia, while at the same time comparing the analysis results between those using the Prominence-i (with the standard configuration or with the delay volume-compatible system kit) and those obtained using both the conventional integrated LC-2010 model as well as a third-party company's LC system. In addition, we introduce an example of method transfer from that used with Shimadzu's conventional system or that of a third-party LC system, respectively. At the same time, we introduce an example of high-speed analysis using the Prominence-i (with Low-volume tubing kit installed) in which the analysis time is shortened to about one fourth the time, while conforming to the Japanese Pharmacopoeia default of conditions.
Extractables and Leachables Analysis Applications
Thermal Desorption - GC/MS Method for Screening Analysis of Extractables in Drug Packaging Materials
Both extractables and leachables (E&L) from pharmaceutical packaging materials and products are of utmost concerns by authorities, since they may affect the efficacy, quality and safety. Many regulatory guidance documents have been established regarding E&L approach and assessment. However, details on how to perform E&L evaluation in various packaging materials and products is still under discussion and development. Extractables are defined as the compounds that can be extracted from a drug packaging under certain conditions, e.g. in solvent and/or with heating. Meanwhile, leachables are compounds that migrate from the drug packaging into the drug under normal storage conditions. Theoretically, leachables emerge from extractables, although not all leachables are extractables in practice. Analysis methods are needed for the detection and quantitation of extractables and leachables in pharmaceutical packaging and products. Here, we describe a screening analysis method for extractables in the packaging of ophthalmic solution by thermal desorption(TD)-GC-MS. The result is compared with leachables result of ophthalmic solution measured by GC-MS with liquid injection.
Extractables Analysis of Elements in Plastic Pharmaceutical Packaging
The extractables and leachables from packaging into pharmaceuticals is a critical issue that must be addressed. This Application News uses inductively coupled plasma mass spectrometry (ICP-MS) to perform a detailed analysis of packaging extractables that targets the elements listed in the ICH Q3D guideline on elemental impurities in pharmaceuticals. ICP-MS offers a detailed breakdown as it can simultaneously analyze an extracted solution for multiple elements with high sensitivity.
Analysis of Thermal Extracts from an Eye Drop Container via the Trapped Headspace (THS) Method
Compounds eluted from pharmaceutical packaging are transferred to the pharmaceuticals, and the ensuing risk to humans from continuous exposure is a focus of interest. These substances are referred to as extractables and leachables, which together are abbreviated as E&L. Methods for measuring them are being sought at present, and various suggestions have been proposed. When screening for volatile and semi-volatile compounds, high sensitivity results are comparatively easy to obtain with the combination of GCMS and the trapped headspace (THS) method, which is why it is a focus of interest. The HS-20 NX Trap is a trap headspace sampler equipped with an electronic cooling trap. It is optimal for thermal extraction as it can be heated up to 300 °C. In trap mode, which uses the trapped headspace (THS) method, basically the entire volume of the headspace is concentrated into the trap. As a result, in comparison with loop mode, which uses the static headspace (SHS) method, more than 20 times the sensitivity is obtained with THS, which approaches the sensitivity of thermal desorption (TD). This articles introduces a comparison of the measurement results for thermal extracts from an eye drop container using SHS, THS, and TD.
Analysis of Extractables from Pharmaceutical Packaging Materials by Solvent Extraction-GCMS and Headspace-GC-MS
Mutual interactions between the pharmaceutical and packaging are a problem with pharmaceutical packaging materials that requires particular care. Extractables are compounds generated from storage conditions that are more severe than normal, whereas leachables are compounds that can be transferred from the packaging to the pharmaceutical under normal storage conditions. They are classified as indicated in Table 1. Before pharmaceuticals can be sold, extractables and leachables must be comprehensively verified to determine packaging material risks.
TOC – Determination according to USP 661.1 Testing of Plastic Packaging Systems and their Materials of Construction
Plastic packaging systems for pharmaceutical products must be suitable for their intended use. The US Pharmacopeia revised the related chapter. It is published in USP 39- NF34, which will be valid from May 2016. Besides to the change of the title “Plastic Packaging Systems and their materials of Construction”, two new chapters are established. This application note is related to the first chapter 661.1.
TOC – Determination according to USP 661.2 Testing of Plastic Packaging Systems and their Materials of Construction
In the pharmaceutical industry plastic packaging is used in various forms – for example for intravenous bags, bottles, cartridges or pre-filled syringes. The packaging must be tested for suitability for these uses. It is published in USP 39-NF34, which will be valid from May 2016. Besides to the change of the title “Plastic Packaging Systems and their materials of Construction”, two new chapters are established. This application note is related to the second chapter 661.2.
News / Events
-
AAPS Pharm Sci 360 2025
November 9-12
Henry B. Gonzalez Convention Center
San Antonio, TX -
Pharma Community Network Event
Shimadzu Scientific Instruments and ZefSci invite you and your analytical teams to a pharma community networking event to celebrate 150 years of science and innovation at The Foundry & Lux in South San Francisco. Our event will be built around innovation, collaboration, and connection.
-
ASMS (American Society for Mass Spectrometry) 2025
June 1-5
Baltimore Convention Center
Baltimore, MD -
Shimadzu Scientific Instruments Opens Boston Location of Its R&D Center Focus will be on promoting customer-oriented development to expand business in the pharmaceutical field
Shimadzu Scientific Instruments, Inc. (SSI, Columbia, Maryland, USA), a Shimadzu Group company, has opened a satellite lab in Boston, Massachusetts to be the base of its collaborative research and development activities on the East Coast. Established to conduct research and development more closely linked to customers, SSI's R&D Center consists of three bases, with the main facilities at its Maryland headquarters, a West Coast location, and this new space in Boston near the city center. The Boston lab was set up by partnering with Labshares, a shared laboratory service provider for life science companies.