Solutions for Alcohol-based Hand Sanitizer Flyer (PDF)
Hand Sanitizers and Disinfectants Analysis of Alcohols

As a result of the global Covid-19 pandemic, use of alcohol-based hand sanitizers and disinfectants has increased dramatically. As with any over-the-counter pharmaceutical product, care must be taken to verify its potency and purity to ensure safe, on-label use.
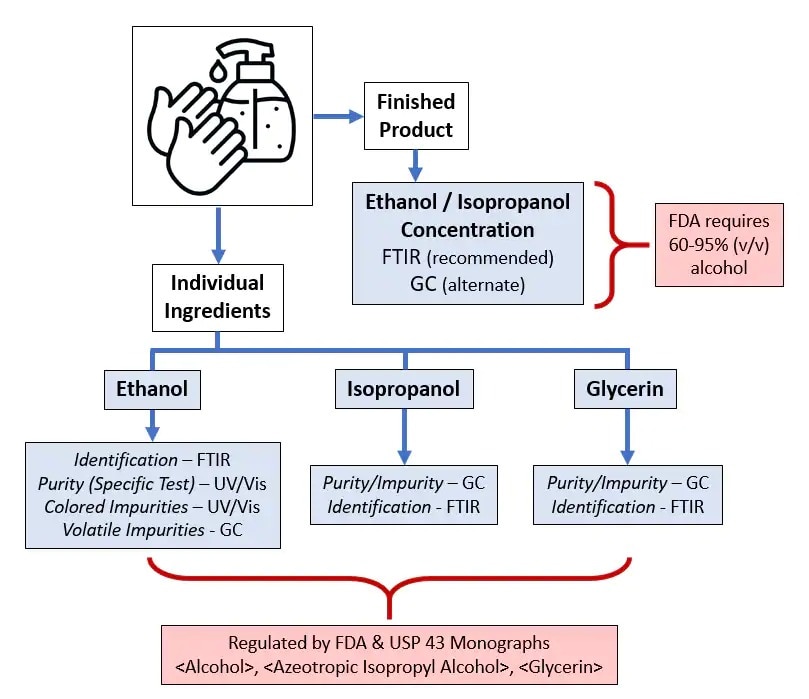
Shimadzu provides all of the analytical solutions necessary for testing of hand sanitizers and their components, including USP/FCC ethanol and isopropanol, neutral spirits, and others by techniques such as GC, FTIR, and UV-Vis.
Analytical Solutions
Hand Sanitizers: Which Analytical Technique is Right for Me?
Analysis for Volatile Trace Contaminants by Gas Chromatography
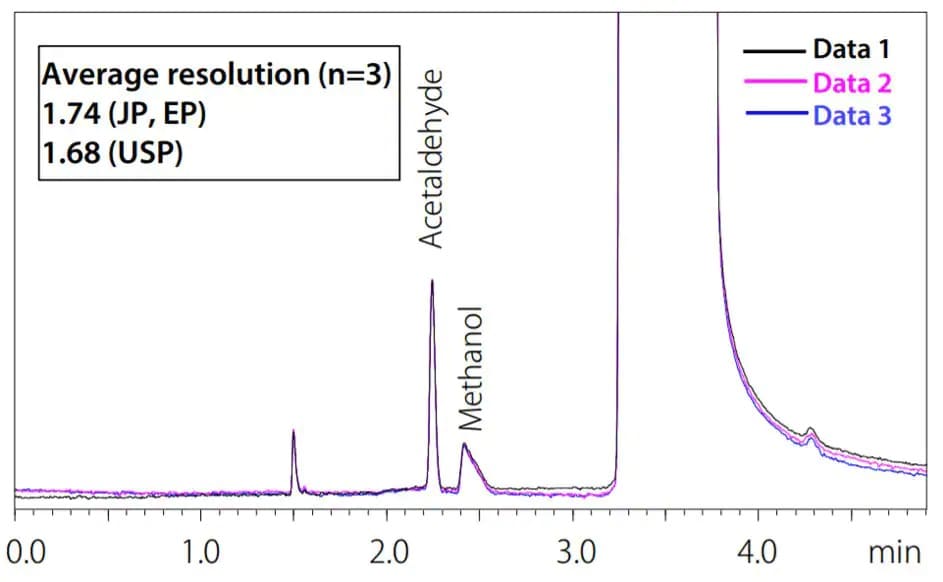
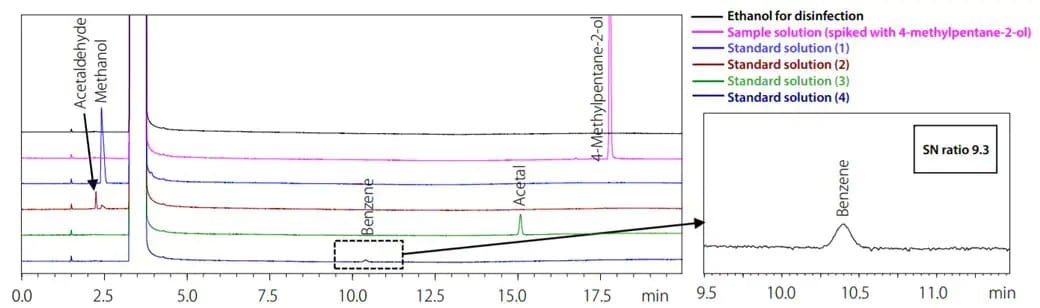
Volatile impurities in ethanol and isopropanol such as methanol, acetaldehyde, acetal, and benzene in ethanol are measured with a gas chromatograph. With an appropriate instrument configuration, separation of acetaldehyde and methanol is sufficient to meet resolution specifications set forth in various pharmacopeial regulations, including the USP as well as JP and EP.
Determination of Alcohol Concentration by FTIR and GC
The US Food and Drug administration states that hand sanitizers must contain at least 60% alcohol to be effective for germicidal and viricidal properties.
Using FTIR with a Q-ATR prism allows for fast, repeatable analyses to ensure that alcohol and hand sanitizer formulations are of the appropriate concentration.
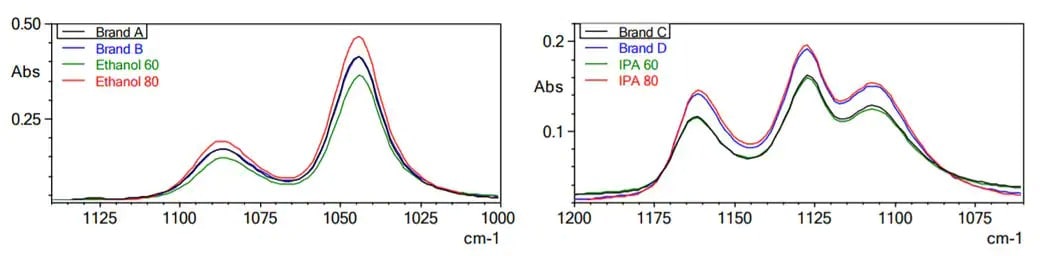
FTIR spectra for ethanol concentration (left) and isopropyl alcohol (IPA) concentration (right).
Alternatively, gas chromatography can be used to determine the ethanol and isopropanol concentrations in finished hand sanitizers. We have developed a method to analyze for alcohol concentrations from 60% to 100% in finished products using nitrogen carrier gas for fast, cost-effective analysis.
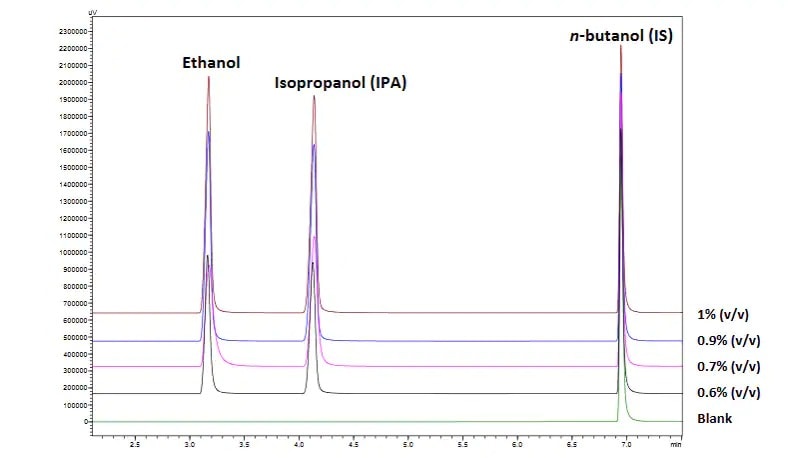
Stacked chromatograms of ethanol and isopropanol concentrations between 0.6% and 1% after 100× dilution.
Determination of Alcohol Purity by UV-Vis Spectroscopy
UV-Vis spectroscopy is used for assessing the purity of alcohols by ensuring absorbances at specific wavelengths are within USP specification and the spectrum must also show a steadily descending curve with no observable peaks or shoulders.
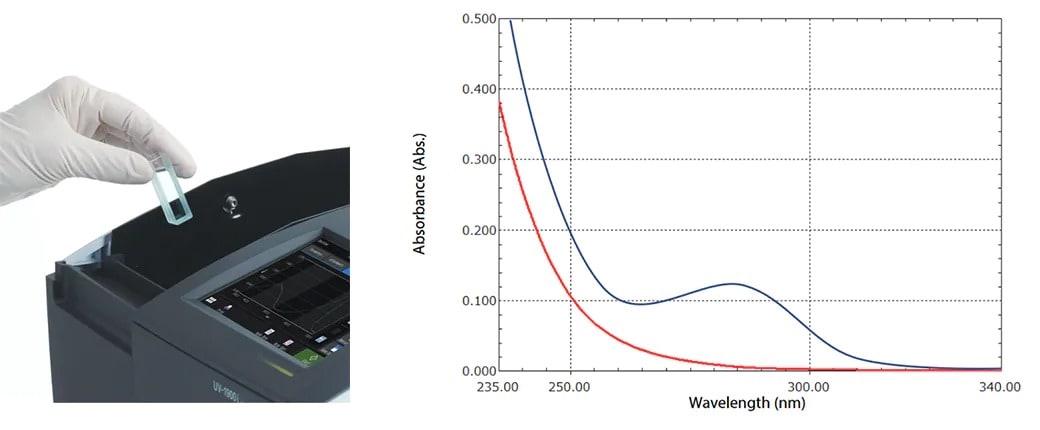
UV-Vis spectra showing acceptable ethanol sample (red) and a rejected sample (blue) on the basis of UV-Vis absorbance.
Relevant Links
A number of governmental agencies and standards organizations are offering free, open access to guides and standards relevant to Covid-19, including hand sanitizers and their formulation. We have included links to pertinent information for your reference: