Electronic theory of Near Infra-Red (NIR) chemistry?
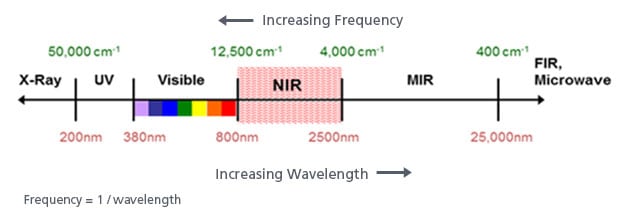
As can be seen from the graphic at the top, both the mid infra-red and near infra-red spectral regions are at longer wavelengths (lower energy) than UV/Visible light. This energy is sufficiently lower that electron promotions do not result from it. But while electrons are not promoted, chemical bonds can be affected.
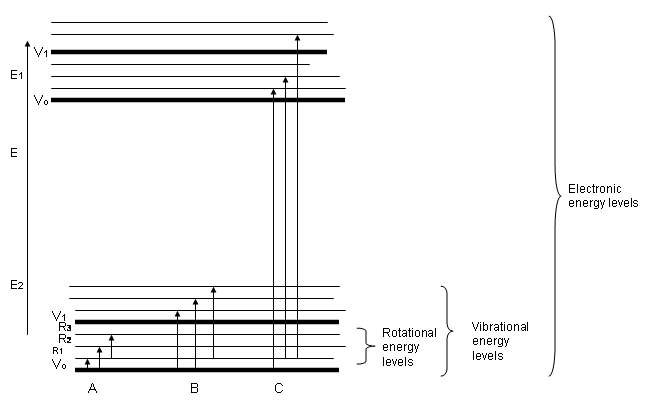
The energy diagram above shows the rather large energy differences between bonding electronic transitions verses the bond deforming vibrational and rotational energy levels caused by mid infra-red frequencies. As such, infra-red spectroscopy yields information on what atoms are bonded to other atoms in a molecule. This is very useful for materials identification and characterization.


