Measurement Methods for Liquid Samples
■ Introduction
This is the second page introducing the measurement methods to adopt for a specific sample form. It introduces the measurement methods for liquid samples. For infrared spectroscopy, liquid samples are easier to handle than solid or gas samples. The page below describes common transmission methods (liquid membrane method and solution technique) and ATR method that has now become easy due to the widespread adoption of FTIR.
■ Measurements by Transmission Methods
Various cells are used for transmission measurements. The types of cell and their applications are introduced below.

Fig. 1 Appearance of a Liquid Cell
(1) Liquid Cell
The liquid membrane method involves dripping several drops of the sample onto an NaCl or KBr aperture plate and sandwiching it under another aperture plate, such that no gas bubbles are trapped. The thickness is adjusted according to the sample absorbance by inserting spacers between the aperture plates or by appropriately tightening the screws (without breaking the aperture plates). This type of cell is called a "liquid cell." Fig. 1 shows the appearance of a liquid cell. This type of cell is also called a "demountable cell" because the holder, aperture plates, and spacers can be disassembled and then re-assembled to perform measurements. The measured sample forms a thin liquid membrane between the two aperture plates, so that this cell type can easily measure high-viscosity liquids. As the thickness is not constant from measurement to measurement, this type of cell is unsuitable for quantitative analysis, except for the internal standard method. These cells are also unsuited to low-boiling-point samples that may vaporize during measurement.
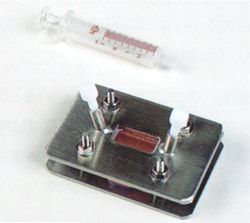
Fig. 2 Appearance of a Fixed Cell
(2) Fixed Cell
A fixed cell, as shown in Fig. 2, is used to measure a volatile sample or a solution of a sample dissolved in an appropriate solvent. A fluororesin stopper at the sample inlet allows sample introduction and extraction from the cell and cell washing without disassembling the cell. As the fixed cell is assembled to be leak-free incorporating a spacer of constant thickness between the aperture plates, it is suitable for quantitative analysis and the measurement of volatile samples. The appropriate cell thickness must be selected for the absorption intensity of the measured sample. However a cell of about 0.1 mm thickness is commonly used to permit easy sample introduction and extraction.
(3) Other Cells
As its name suggests, a fixed cell has a fixed thickness. If a variable thickness is required, an air-tight liquid cell or variable cell can be used. The air-tight liquid cell is assembled by the user, using a spacer of appropriate thickness. The thickness of a variable cell can be continuously adjusted while the thickness is indicated on a scale. The cell thickness can be adjusted from 0.01 to 5 mm. Small-capacity cells, called microcells or ultra-microcells, are also available to handle extremely small sample volumes. Oil-measuring cells with a long light path length of 1, 5, or 10 cm are available to measure oils extracted in a solvent such as carbon tetrachloride.
Table 1 summarizes the applications of liquid cells and fixed cells.
Table 1 Applications of Liquid Cells and Fixed Cells
| Name | Purpose | Quantitative analysis |
| Liquid cell (Demountable cell) |
• Non-volatile liquids (high-boiling-point, high-viscosity oils, etc.) • Qualitative analysis of samples by Nujol method, film method, etc. • Thickness adjustable using spacers or tightening the screws |
Partially suitable |
| Fixed cell | • Volatile liquids (chloroform, etc.) • Components in solution • Fixed thickness |
Suitable |
■ Cell Materials
After selecting the appropriate cell type from Table 1, aperture plates must be selected to suit the sample. The aperture cell materials described here are commonly used to measure liquid samples.
Alkali halides, such as NaCl, KBr, or CsI, are often used due to their good infrared transmission. However, these materials are highly deliquescent and not resistant to moisture. Consequently, such aperture plates cannot be used with solutions containing water or low-grade alcohol. KRS-5 (thallium bromide / iodide crystal) aperture plates are comparatively resistant to water. However, they are slightly soluble in water and can only be used for a short time. Arsenic selenide (As2Se3) aperture plates used to be commonly used, as they are insoluble in water. However, they have a high refractive index and low transmittance, and are easy to break. Zinc selenide (ZnSe) has recently attracted attention as a material for aperture plates. While these aperture plates are rather expensive, they are insoluble in water, have a lower refractive index than As2Se3, and are easy to use. The measured wavenumber range must also be considered when selecting the aperture plates. For example, as NaCl offers transmission only down to 650 cm-1 on the low-wavenumber side, it cannot be used to obtain a spectrum across the 600 to 400 cm-1 range. Therefore, when selecting aperture plates, be aware of (1) the sample properties, (2) the measured wavenumber range, and (3) the price. Table 2 summarizes the characteristics of aperture plates that are commonly used in liquid cells.
Table 2 Types and Properties of Aperture Plates
| Aperture plate material | Transmission wavenumber range (cm-1) | Solubility in water and other properties |
| NaCl | 28000 - 650 | Soluble, hard |
| KBr | 33000 - 400 | Soluble |
| KRS-5 | 14000 - 330 | Almost insoluble, orange color, toxic |
| Csl | 33000 - 140 | Soluble, soft |
| AS2Se3 | 10000 - 625 | Insoluble |
| ZnSe | 20000 - 500 | Insoluble, orange color, soft |
■ Attenuated Total Reflection (ATR) Method
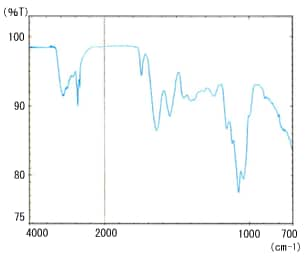
Fig. 3 Milk – Pure Water Difference Spectrum
Water is the commonest liquid around us. It has an extremely strong absorption peak in the infrared range. This absorption peak is so strong that is cuts out the infrared light even if a 0.025 mm spacer is used. Therefore, measuring a water solution requires an even thinner liquid membrane or replacement of the hydrogen with deuterium to shift the absorption peak toward the low wavenumber end.
As FTIR becomes more widespread, ATR method is a method that is once more attracting interest for its utility. ATR method is proving to be an extremely effective method, even for water solutions, which have been difficult to measure with other FTIR methods. It can be thought of as a method that only measures the extremely thin layer in close contact with a prism. Liquids are ideal samples for ATR method, as they form close contact with the prism. The definition of "extremely thin layer" differs according to the prism material but is approximately 1/5 to 1/10 the light wavelength. This equates to 1 to 2 µm (0.001 to 0.002 mm) for 1000 cm-1 (10 µm) light. Therefore, the light can be transmitted even through water, which exhibits strong absorption.
Prisms are available in ZnSe, Ge, and ZnS materials. The cylinder internal reflection type ATR attachment is also available to support the application of ATR method for minute sample volumes and flows. Fig. 3 shows the spectrum of commercial milk by ATR method. It is a difference spectrum, with the absorption of water subtracted from the milk spectrum. A good spectrum is obtained, even at the strong absorption peaks for water near 3400 and 1650 cm-1.
■ Conclusions
The measurement methods for liquid samples are outlined above. FTIR measurements are comparatively simple to perform but the cell type and aperture plates must be selected according to the sample and the purpose of analysis.


