Light Sources for Spectrophotometers
In this issue, we will describe the light source, an important part of the spectrophotometer that was explained in "The Structure of a Spectrophotometer" in UV Talk Letter Vol.2.
1. Light Source Requirements
The requirements for a spectrophotometer light source include:
a) bright across a wide wavelength range;
b) stable over time;
c) long service life; and
d) low cost.
"a) Bright across a wide wavelength range" demands both a high degree of brightness and uniform brightness across the measurement wavelength range (uniform brightness distribution).
"A high degree of brightness" is a necessary requirement to obtain photometric values with a high S/N ratio. However, as increasing the brightness of the light source generally results in a reduction in condition "c) long service life," the balance between the two must be considered.
As the sensitivity characteristics of the detector are also relevant during actual measurements, a "uniform brightness distribution" is an important condition for acquiring photometric values with a uniform S/N ratio within the measurement wavelength range.
2. Types of Light Source
Many light sources meet some of the requirements above but no light source is able to meet them all. Many spectrophotometers switch between a halogen lamp for the visible range and a deuterium lamp for the ultraviolet range according to the wavelength setting.
This is because of the difficulty in achieving both "a high degree of brightness" and a "uniform brightness distribution" across a wide wavelength range using a single light source. Switching between light sources with different emission wavelength ranges also offers the advantages of reducing the excess incident light into the monochromator and reducing the amount of stray light (Reference 1).
Other instruments use a xenon lamp or xenon flash lamp suitable for the analysis target and aim of the analysis. A lowpressure mercury lamp that produces multiple emission spectra is effective for spectrophotometer wavelength calibration.
| Reference 1 : What is stray light? |
| Stray light is defined as the ratio of the total light intensity at wavelengths other than the set wavelength with respect to the light intensity emitted from the wavelength selection device (from JIS K 0115). For example, assume that 1 % transmittance (2 Abs) was measured at a certain wavelength when monochromatic light containing 0.1 % stray light was incident on the sample. Due to the effect of the stray light, the transmittance is actually 1.1 % (1.959 Abs), such that an error of approximately 2 % occurred. |
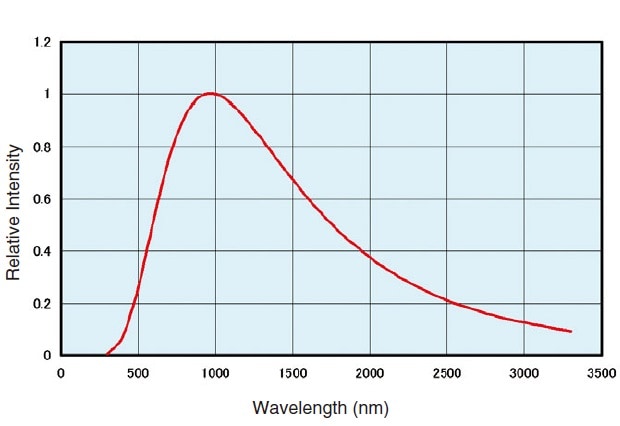
Fig.1 Emission Intensity Distribution of a Halogen Lamp(3000K)
(1) Halogen Lamps
Similar to a normal incandescent lamp, a halogen lamp filament heats up and emits light when a current flows through it. The tungsten used as the filament material evaporates at high temperatures. Consequently, the bulb containing the filament of a normal incandescent lamp is filled with an inert gas to prevent evaporation of the tungsten.
A halogen lamp contains a halide as well as the inert gas to create the halogen cycle (Reference 2) that returns evaporated tungsten to the filament, resulting in a long lamp life. It also restricts blackening of the tube wall, due to adhering evaporated tungsten, to create a light source that remains bright over long periods.
Fig. 1 shows the light intensity distribution at 3000 K color temperature. The usable wavelength range is 350 nm to 3500 nm, but this is affected by the color temperature.
Halogen lamps are stable over time, offer a long service life (approx. 2000 hours) and are relatively cheap. As such, they many of the conditions required for a spectrophotometer light source.
| Reference 2 : What is the halogen cycle? |
| Tungsten that evaporates at high temperatures binds with halogen near the cool tube wall to form tungsten halide. The suspended tungsten halide moves inside the tube due to convection and separates into halogen and tungsten near the hot filament. The separated tungsten adheres to the filament and the halogen bonds again with evaporated tungsten. This repeated reaction is known as the tungsten cycle. |
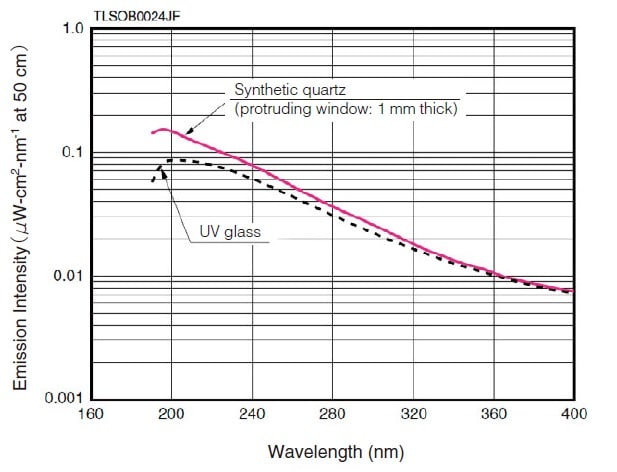
Fig.2 Emission Intensity Distribution of a Deuterium Lamp 1)
(2) Deuterium Lamps
A deuterium lamp is a discharge light source with several hundred Pa deuterium sealed in a bulb. As it uses a hot cathode to achieve stable and reliable arc discharge, approximately 10 sec for preheating is required before starting the discharge.
A deuterium lamp requires a large and complex power supply, making it more expensive than a halogen lamp. However, it is one of the few continuous spectrum light sources that is stable in the ultraviolet range.
The deuterium lamp has a short emission wavelength of 400 nm, or less. The window material limits its use at the short wavelength end.
Fig. 2 shows examples using synthetic quartz and UV glass.
The use at the long-wavelength end is limited to about 400 nm. However, the low degree of attenuation toward the longwavelength end permits use of light above 400 nm. Multiple emission spectra also exist in the range at 400 nm and above. Of these, the spectra at 486.0 nm and 656.1 nm are particularly strong (see Fig. 5) and can be used for wavelength calibration of the spectrophotometer.
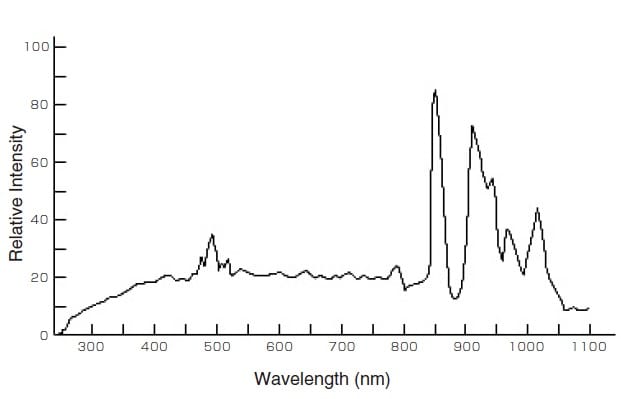
Fig. 3 Emission Intensity Distribution of a Xenon Lamp 2)
(3) Xenon Lamp (Xenon Arc Lamp)
A xenon lamp is a discharge light source with xenon gas sealed in a bulb. Xenon lamps are categorized as directcurrent or alternating-current types, according to the lighting method. If the electrodes become too hot, the tungsten electrode material can evaporate and adhere to the tube wall, resulting in a loss in brightness. As the anode becomes particularly hot, the anode of a direct-current type xenon lamp is made larger than the cathode to increase its thermal capacity. As the electrodes of an alternating-current type electrode alternately become the cathode and anode, both electrodes are the same size. Therefore, the tungsten evaporates more easily than with the direct-current type. However, the alternating-current type permits the use of a compact, low-cost lighting device, as no current rectification is required.
The xenon lamp exhibits a similar spectral distribution to sunlight and produces a continuous spectrum from the ultraviolet to the near-infrared, as shown in Fig. 3. Overall, the xenon lamp is inferior to the halogen lamp and deuterium lamp in terms of cost and output fluctuations. Halogen lamps are often used in general spectrophotometers but xenon lamps are used in cases where a high light intensity is required (such as spectrofluorophotometers), due to their high brightness.
(4) Xenon Flash Lamp
This is a compact xenon lamp that generates little heat due to pulsed ignition. Straight and U-tube types are available, depending on the application. The electrodes are sealed in a quartz glass tube (or high-silica glass tube) that is filled with xenon gas.
However, because of its poor reproducibility, a result of larger output fluctuations as compared to an arc lamp, integration of the output data is required to obtain stable data. Therefore, it is used in combination with an array detector in automated instruments (such as colorimeters) to rapidly obtain continuous spectra.
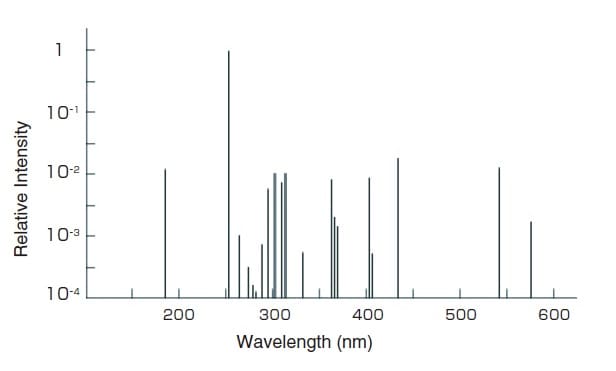 Fig.4 Spectral Distribution of a Low-Pressure Mercury Lamp 3)
Fig.4 Spectral Distribution of a Low-Pressure Mercury Lamp 3)
(5) Low-Pressure Mercury Lamp
The low-pressure mercury lamp is a discharge lamp designed to have a low mercury vapor pressure (100 Pa max.) when lit to efficiently emit the mercury resonance lines (254 nm or 185 nm).
Fig. 4 shows the spectral distribution of a low-pressure mercury lamp.
Low-pressure mercury lamps are available in versions that use the emitted ultraviolet lights directly, or as so-called fluorescent lamps that use a fluorescent material to convert the wavelength to a different wavelength.
A spectrophotometer uses the mercury emission lines to calibrate the displayed wavelength values. The 254 nm, 365 nm, 436 nm, or 546 nm emission lines can be used for the calibration but care is required with the slit width (spectral bandwidth) used during measurements. For example, as the 365 nm emission line is a triple line (three emission lines in close proximity), the spectral bandwidth must be 0.5 nm max. to accurately measure the respective emission lines.
3. Switching Light Sources
As stated above, halogen lamps and deuterium lamps are used in many spectrophotometers.
Fig. 5 shows their respective energy distributions measured by a UV-1800 UV-VIS Spectrophotometer. The light sources are switched near 300 nm to 350 nm, where the emission intensities of the halogen lamp and deuterium lamp are approximately equal.
The light sources can be switched by moving the lamps themselves or by rotating a reflector.
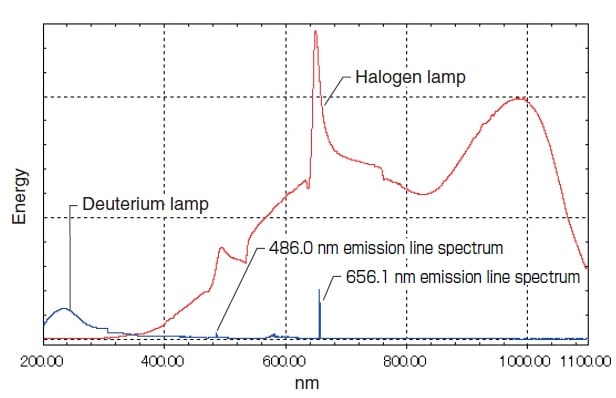
Fig. 5 Light Source Energy Distribution Measured by the UV-1900
Fig. 6 shows the switching method by rotating a reflector. By changing the tilt of the reflector positioned between the halogen lamp and the deuterium lamps, the light beam that enters the monochromator can be switched.
In the UV-1900, the optimal tilt with respect to each light source is automatically adjusted during the initial setup operation after the power is turned on, which eliminates the need for positional adjustment by replacing lamps.
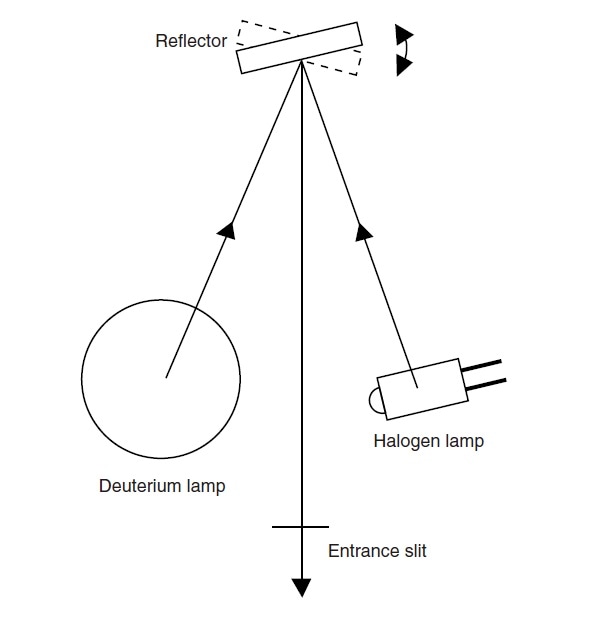
Fig. 6 Method for Switching Light Sources
4. Conclusions
This article focuses on the light source, one element of the spectrophotometer. It described typical lamp characteristics and switching mechanisms.
- 1) Hamamatsu Photonics K.K. General Lamp Catalog
- 2) Ushio Inc. General Discharge Lamp Catalog
- 3) Ushio Inc. General Discharge Lamp Catalog


