Full View of EI Process
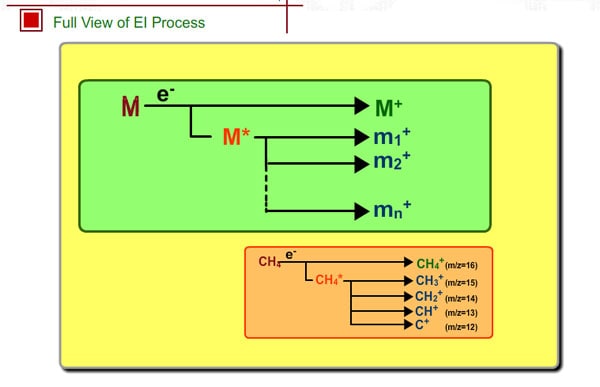
It is said that either a stable molecular ion M+ or an unstable molecular ion M+* is produced by electron impact, the result depending on compounds. For example, straight-chain hydrocarbons with many carbons rarely show molecular peaks, especially for the compounds having many carbons, because the unstable molecular ion M+* easily decomposes into a fragment ion.
For methane, the molecular ion is CH4+. If hydrogen is removed, the fragment ion CH3+ is formed. Formation probability of each ion is constant, so we can get a reproducible spectrum as long as the analytical conditions are the same.
Next >> Producing EI Mass Spectrum


