HPLC vs UHPLC - How to Choose?
High Performance Liquid Chromatography (HPLC) Basics
Introduction to HPLC
High Performance Liquid Chromatography (HPLC) is a process of separating components in a liquid mixture. A liquid sample is injected into a stream of solvent (mobile phase) flowing through a column packed with a separation medium (stationary phase). Sample components separate from one another by a process of differential migration as they flow through the column.
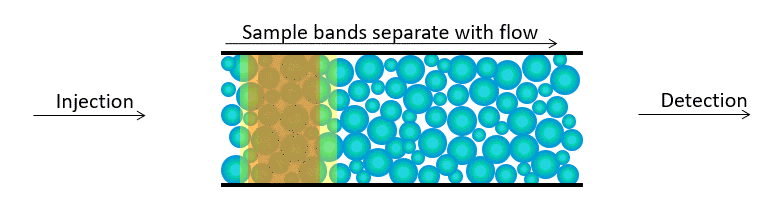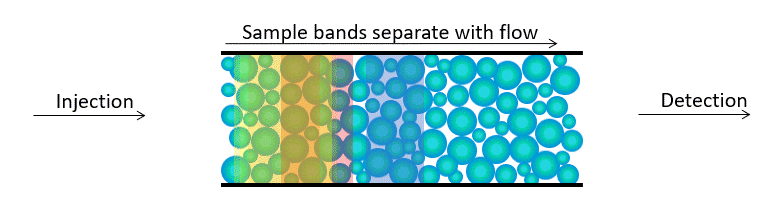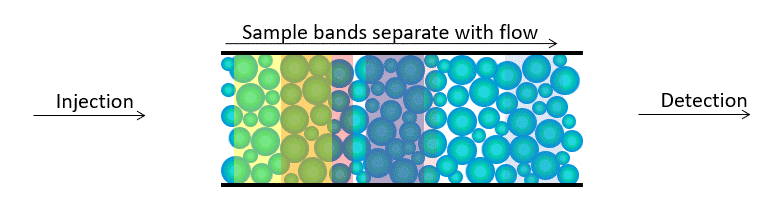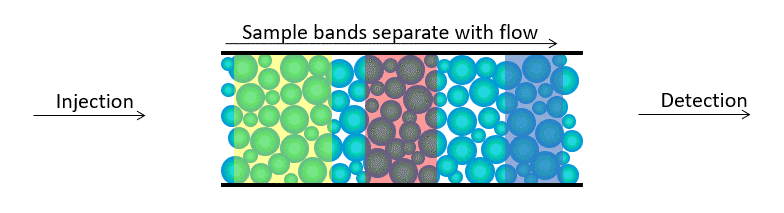
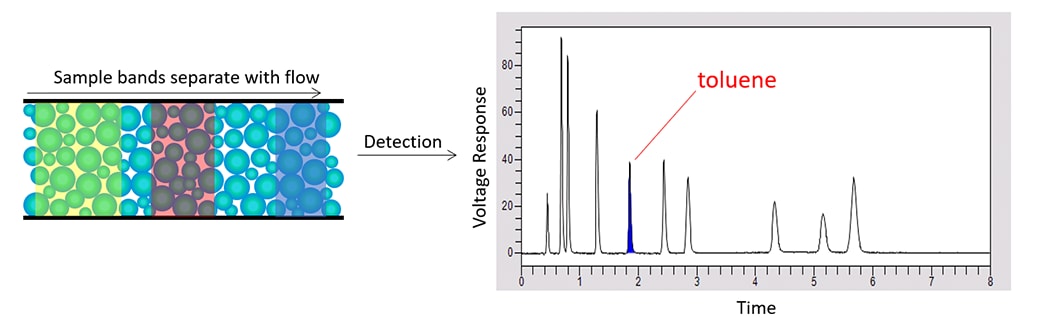
As bands emerge from the column, flow carries them to one or more detectors which deliver a voltage response as a function of time. This is called a chromatogram. For each peak, the time at which it emerges identifies the sample constituent with respect to a standard. The peak’s area represents the quantity.
Basic HPLC System Components
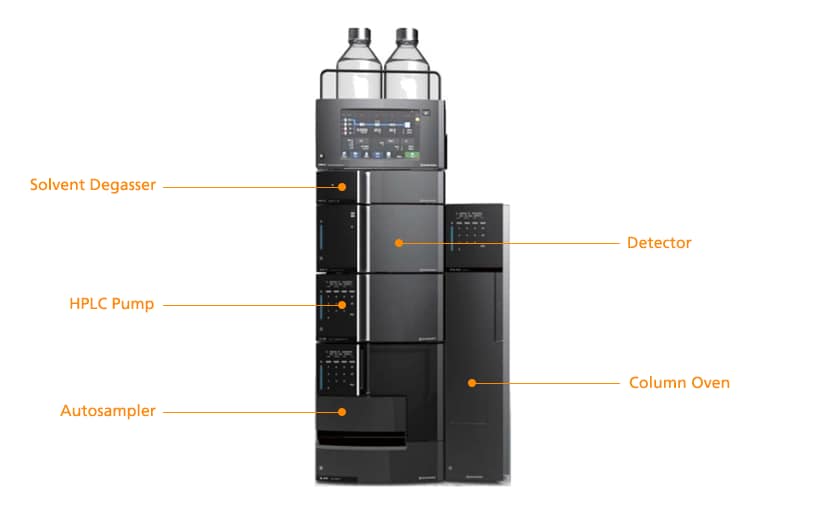
Solvent Degasser – removes air gases from the solvents as they flow to the HPLC pump
HPLC Pump – provides solvent flow and proportioning
Autosampler – draws samples from vials and injects them into the solvent flow provided by the pump.
Detector – responds to the separated analytes emerging from the HPLC column and produces a signal output for the software
Column Oven – houses the HPLC column and keeps a stable temperature for reproducible separations
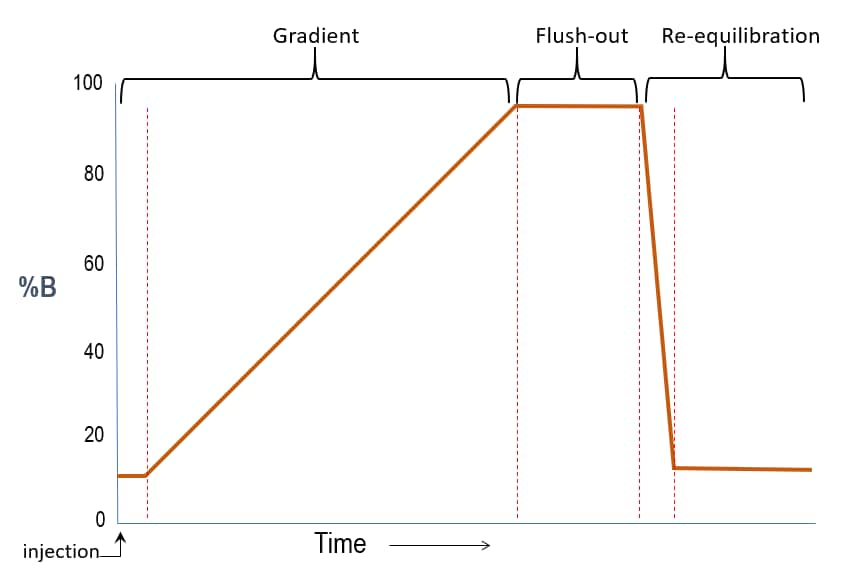
Isocratic vs. Gradient Elution Modes
Isocratic Elution
- A single composition of solvents is used for the duration of the separation
- Later eluting peaks are broader than earlier eluting peaks because of dispersion
- Steps must be taken to periodically flush the column at higher solvent strength to clean it of intractable materials that build up from sample injections
Gradient Elution
- The composition of solvents is changed either continuously or stepwise
- In general, peaks are sharper throughout the chromatogram when compared to isocratic elution
- Some separations may be achieved which are not possible using isocratic elution
- Chromatogram run times may be shorter when compared to isocratic elution
Isocratic and Gradient Elution Pumping Modes
Isocratic Pump
A single channel pump which requires the user to pre-mix the mobile phase. Composition remains constant with time.
Quaternary Low Pressure Gradient Pump
A 4 channel pump which creates mixtures of separate solvent channels under software control. Mixing is done before the pump heads. Composition may be changed with time.
Binary High Pressure Gradient Pump
A 2 channel pump which creates mixtures of 2 solvents under software control. Mixing is done after the pump heads. Composition may be changed with time.
Recommended LC Column:
Column Chemistry: e.g. C18, C8, C4, Phenyl-Hexyl, Biphenyl
Column Format: 100 x 3 mm, 2.7 to 5 µm particles
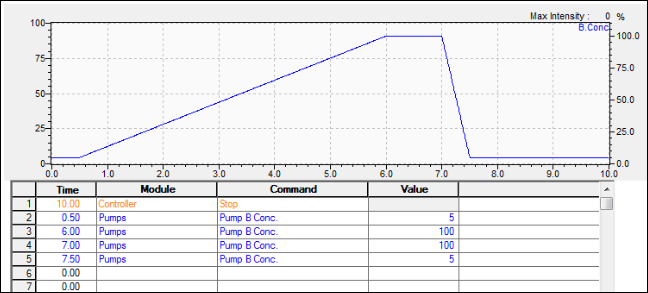
Screening Gradient – Powerful for Method Development
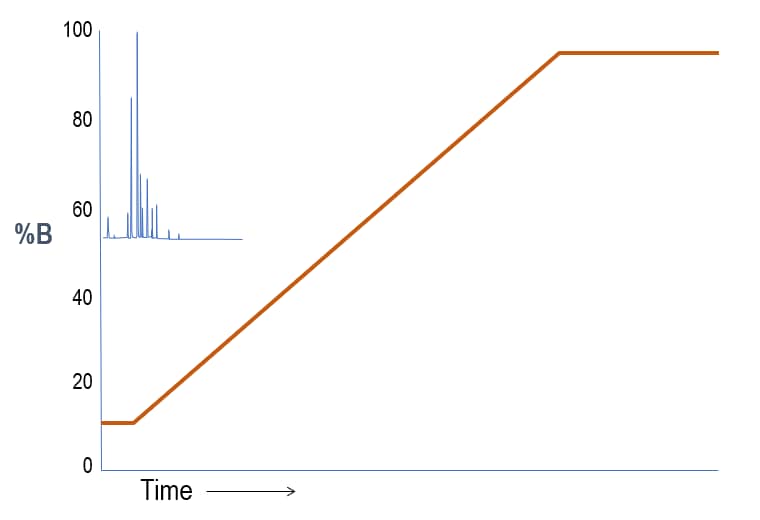
Interpretation: For a reversed phase LC column where A is water and B is organic, this first run shows sample constituents that are all polar. So, the separation is poor because the substances experience little partitioning on the stationary phase. In other words, the weak, starting solvent condition brings the sample constituents off too early.
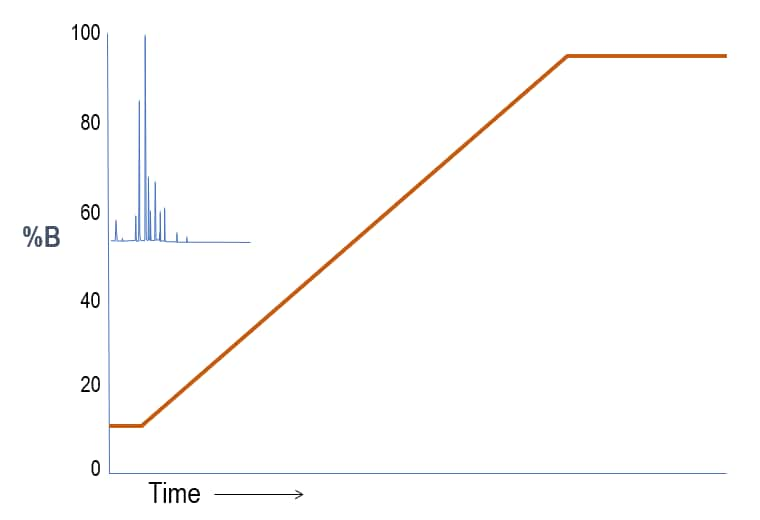
To cause the substances to partition more into the stationary phase, we reduce the slope of the gradient so that the mobile phase strength does not increase as quickly. The substances retain longer and begin to separate from one another. Notice that we still “flush” the column with strong solvent at the end of the run to effectively clean the column.
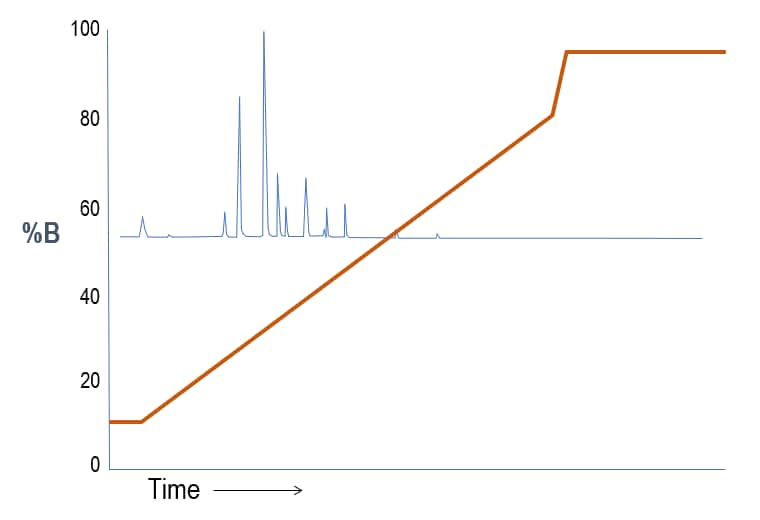
We reduce the gradient slope again until we begin seeing a good separation within the time frame.
Available Detectors for HPLC
UV/Vis Detectors
- Responds to chromophoric analytes in the range 190 – 800nm
- Single wavelength monitoring
- Good for the majority of organic analytes
- Gradient elution compatible
Photodiode Array (PDA) Detectors
- Responds to chromophoric analytes in the range 190 – 800nm
- Multi-wavelength monitoring
- Complete spectral profiles of chromatographic peaks
- Good for the majority of organic analytes
- Gradient elution compatible
>> UV vs Diode-Array (PDA) Detectors for (U)HPLC - a comparision
Fluorescence Detectors
- Responds only to analytes which fluoresce naturally or can be made to fluoresce through derivatization
- Extremely sensitive
- Gradient elution compatible
Refractive Index Detectors
- Considered a universal detector for all analytes
- Comparitively insensitive
- Not compatible with gradient elution
Evaporative Light Scattering Detectors
- Considered a near-universal detector, responding to both chromophoric and non-chromophoric analytes
- Good sensitivity
- Gradient elution compatible
Mass Spectrometric Detectors
- The most discriminating detector
- Delivers mass information for analyte ID and structure elucidation
- Highly sensitive
- Gradient elution compatible
Considerations When Choosing a Column
The decision of a “best” stationary phase for a separation should be based on sample solubility and the chemical differences between the sample constituents.
- Soluble in organic or aqueous solvents?
- Knowledge of constituent functional groups? -OH; -COOH; -CONH2; -C6H6, etc.
The decision about the dimensions of the column should be based on the goals for the chromatography.
- Resolution of a critical pair of constituents? – longer column bed
- Fast analysis? – shorter column bed
- Maximum resolution for all? – smaller particles
- Minimize solvent consumption? – narrower inner diameter
The best choice may be a compromise of different goals!
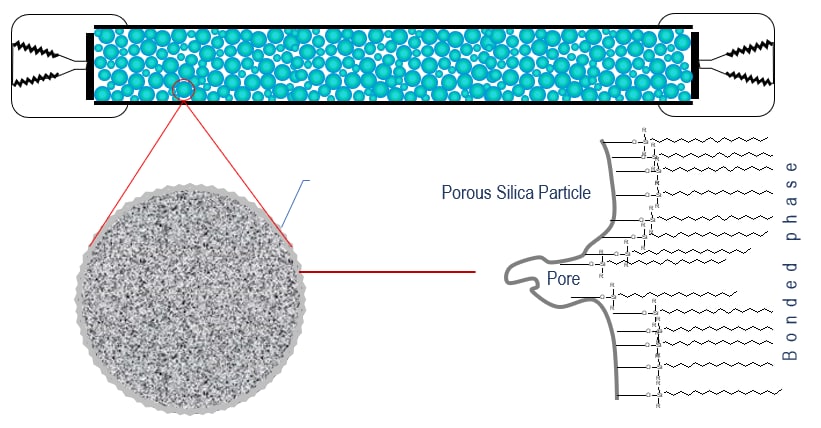
The Chemistry of Bonded Phases
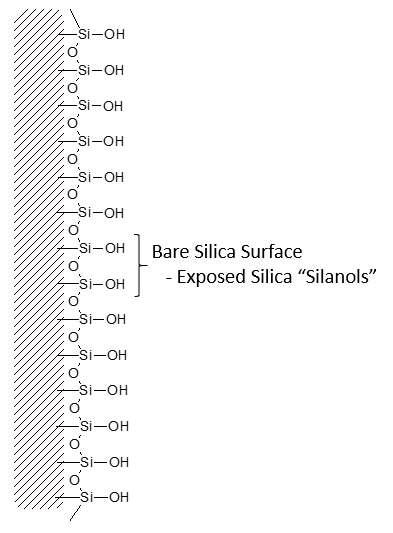
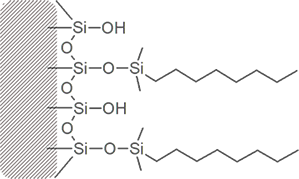
Bonded phase packing media starts with a bare silica surface
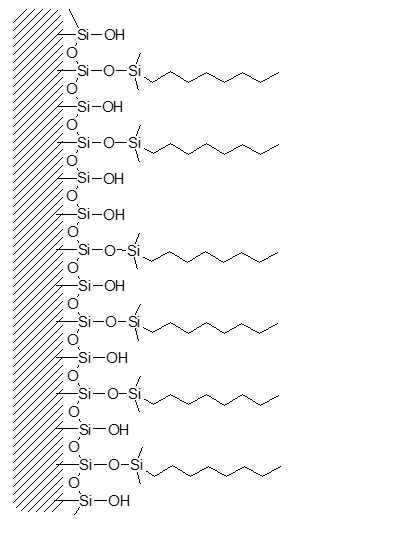
Through chemical reaction, attachment of the primary bonded phase is achieved.
Manufacturers refer to the amount of bonded phase coverage in terms of %Carbon load.
Much of the silica surface remains unreacted – some surface silanol groups are still exposed.
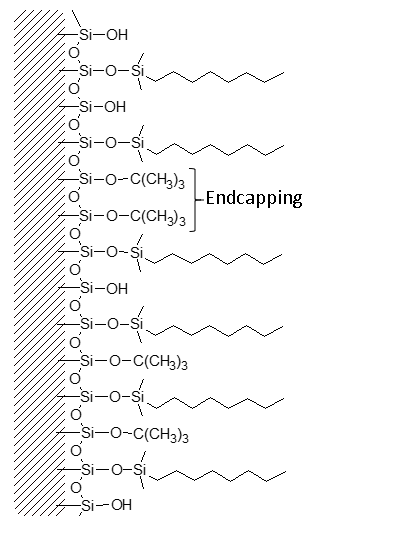
Frequently, a secondary bonding step intruduces smaller “end-cap” molecules between the primary bonded phase strands. These endcaps may be polar or non-polar.
Different columns of the same bonded phase type will differ in silanol exposure and end-capping, resulting in a range of different overall polarities and different separating ability.
-
C18
-
C8
-
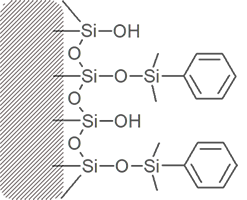
Phenyl
-
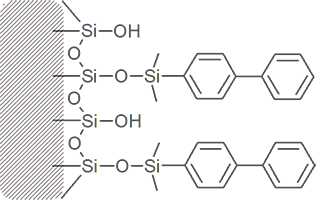
Bihenyl
-
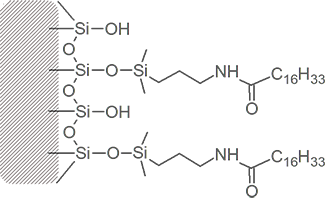
Alkyl Amide
-
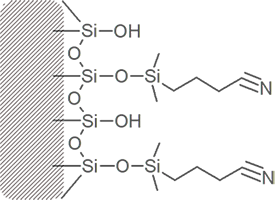
Cyano Propyl
-
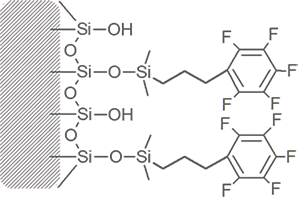
Pentafluorophenyl Propyl
-
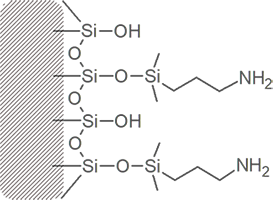
Amino Propyl
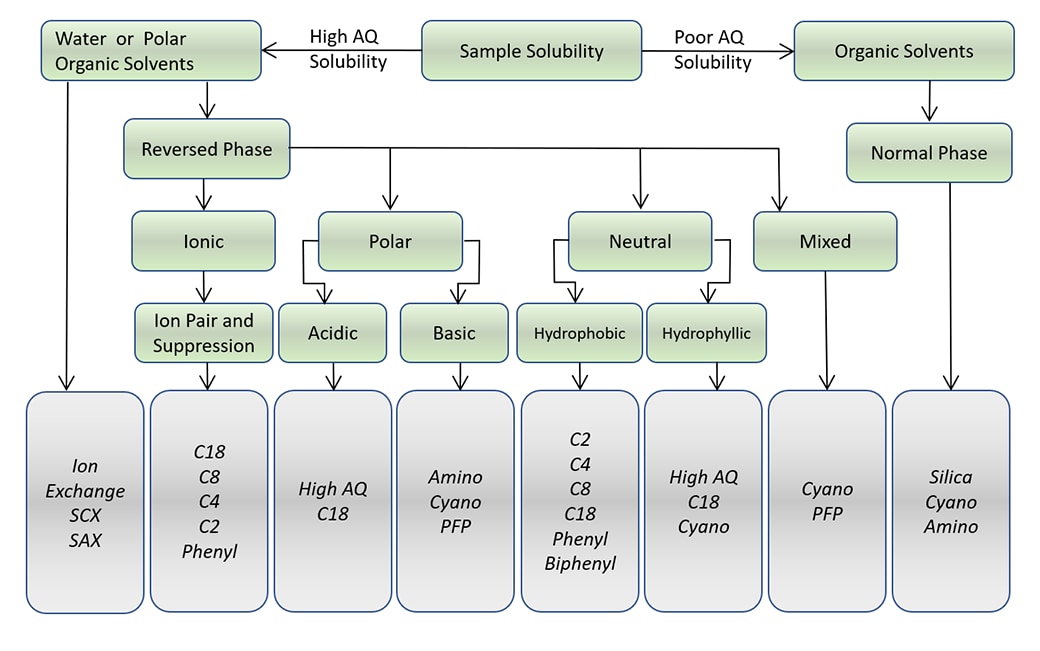
Column Chemistry Choices Based on Sample Solubility
The Phenomenon of Hydrophobic Retention
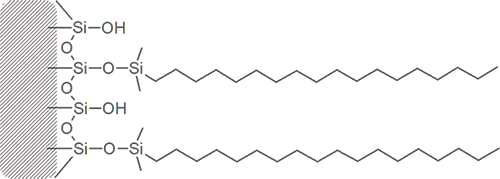
So-called reversed phase columns have a bonded phase on the surface of the silica particles which is hydrophobic in nature. Examples are C30, C18, C8, C4.
In principle, “like dissoves like”. Such a column will tend to retain sample constituents which are also hydrophobic, as long as the mobile phase is not stronger in its attraction for that sample constituent. More polar sample constituents will tend to elute from the column faster because they are retained to a lesser degree.
For a reversed phase column, use a combination of solvents which give an overall “greater” polarity (less hydrophobic) than the stationary phase. In this way, the sample constituents have a reason to be attracted to the stationary phase (column bonded phase) and resist the tendency to flow with the mobile phase (solvent).
Use the “weakest” or most polar condition necessary to differentiate between sample constituents as they migrate through the column.
Examples of common mobile phase solvent pairs are Acetonitrile/Water and Methanol/Water. Changing the ratio of organic/water changes its overall polarity.
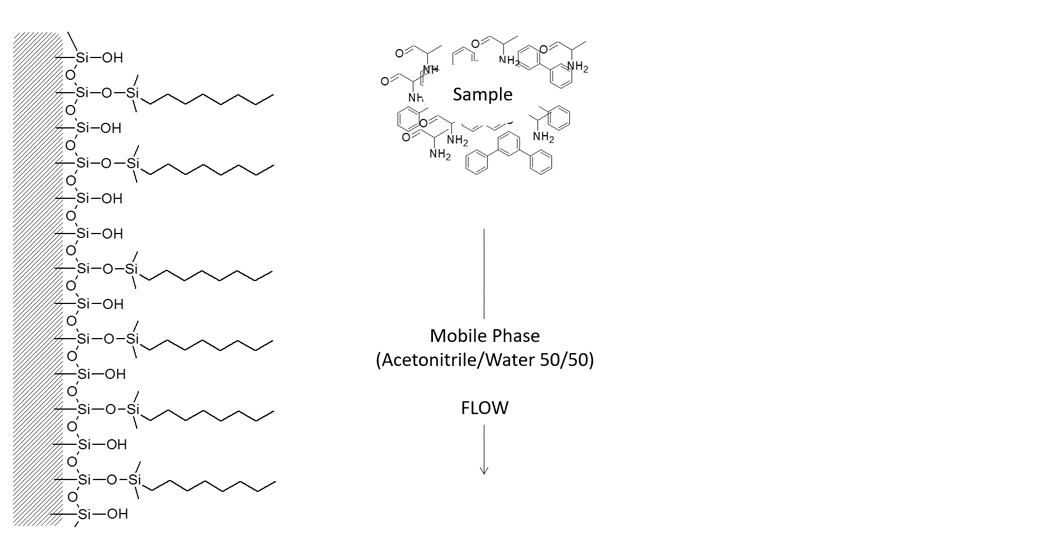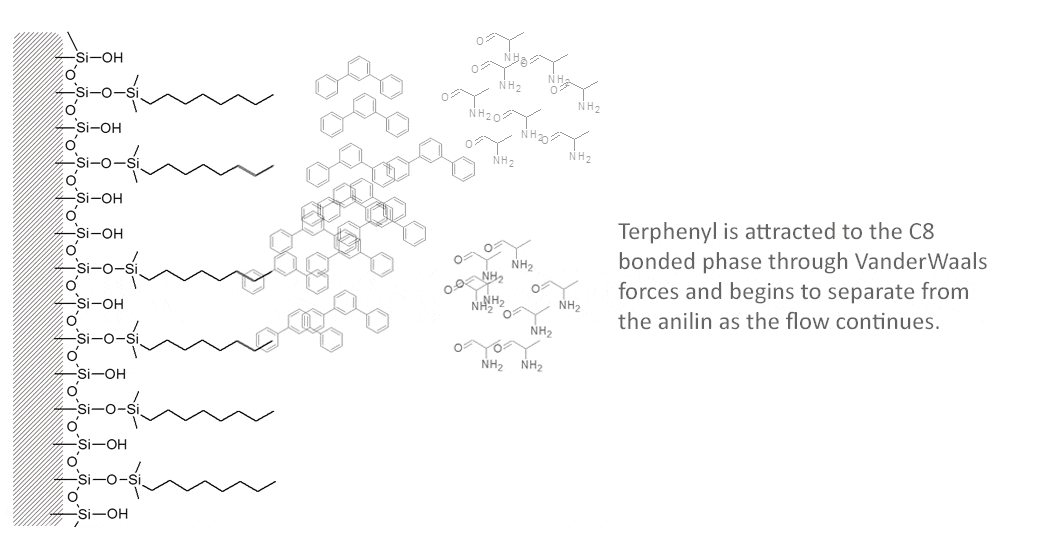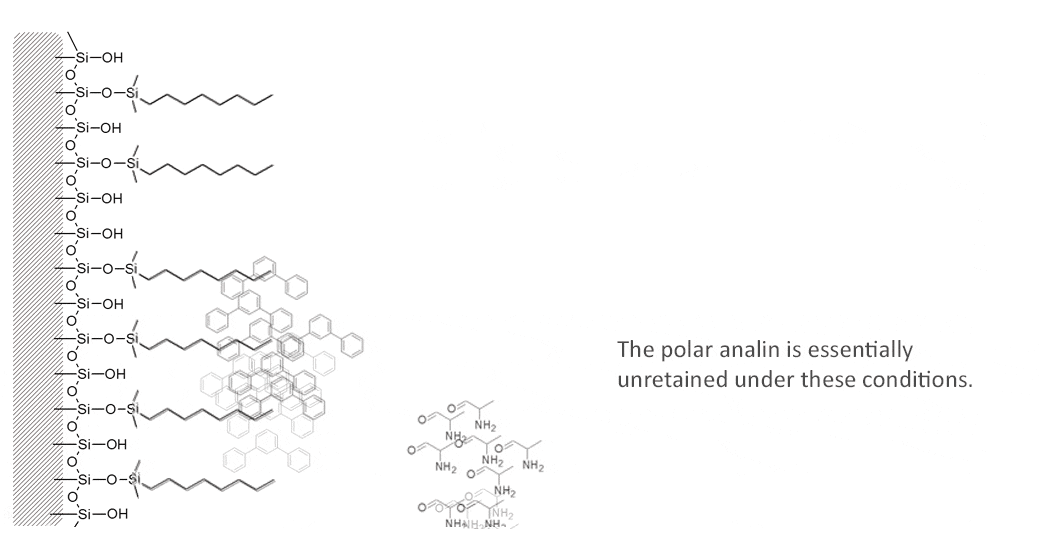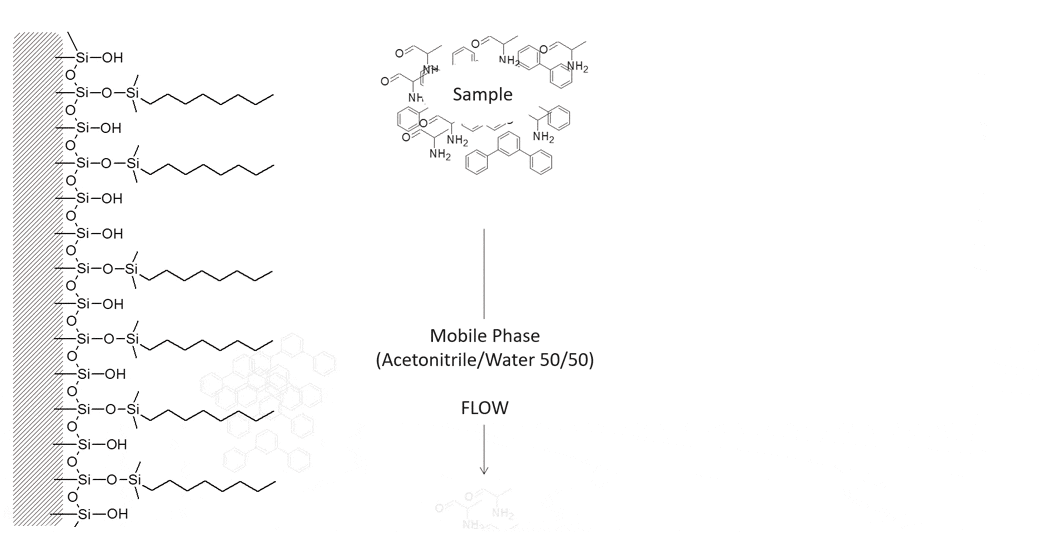
Example of Hydrophobic Retention
The following molecules are easily separated on a reversed phase C8 column using 50/50 Acetonitrile/Water. The polar alanine will elute first from the column followed by the non-polar terphenyl.
-
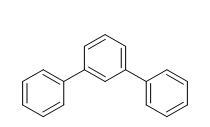
1,1':3',1''-terphenyl hydrophobic or non-polar
-
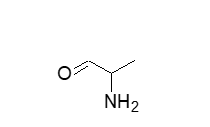
alanine hydrophillic or polar