Request More Information
Monoclonal Antibodies and Biosimilars

Monoclonal antibodies (mAbs) have emerged as revolutionary therapeutic agents for an array of human diseases. In addition to their large mass and complex structure, mAbs are varied in their origin, makeup, effector function and delivery, and therefore require thorough formulation development, including characterization, quantitation and preservation.
More and more special designed Antibody drug conjugates (ADCs) are under development as targeted therapy for treating cancer. ADCs combine the targeting capabilities of monoclonal antibodies with the cancer-killing ability of cytotoxic drugs, allowing new “proximity” based dosing strategies.
As therapeutic development moves from IgG-based antibodies to technologies like bispecific antibodies, peptibodies, and fusion proteins, we are leading innovation in analytical techniques. nSMOL (nano-surface and molecular orientation limited proteolysis) is Shimadzu’s novel approach that enables selective proteolysis of the Fab region of monoclonal antibodies. The nSMOL Antibody BA Kit is a ready-to-use reagent kit for collecting monoclonal antibodies from blood or other biological samples using immunoglobulin collection resin, and then performing selective proteolysis of the Fab region of these antibodies via trypsin-immobilized nanoparticles. Variable region-derived peptides produced by limited proteolysis can then be quantified via MRM measurements utilizing a high-performance liquid chromatograph mass spectrometer.
As first and second generation antibody therapeutics migrate off patent, there is a need to provide the same robust analysis to biosimilar therapeutics. The need to characterize not only the sequence of expressed mAbs, but the various post-translational modifications (PTMs) that can occur is key to proving bioequivalence for regulatory filings. Solutions for N-linked glycan analysis, charge variant analysis, and aggregation state are all available from your partners at Shimadzu.
Analytical Solutions from Cell Culture to Biologics Development and Quality Control
In this study, a multi-instrument platform was used to observe the process of cell culture production to the development of biologics. A CHO cell line was chosen for the study of culture solutions that were sampled every 24 hours and supernatants obtained by centrifugation. An automatic pretreatment with the Shimadzu C2MAP-2030 system was used for removing proteins from culture supernatants by precipitation with organic solvent, suction filtration, and automated transfer of samples for LC-MS/MS analysis (TripleQuad mass spectrometry). In a 20-minute method cycle, the system analyzed 125 metabolites with a wide range of chemical properties simultaneously using high-speed scanning and positive/negative ionization switching. During cell culture, an atomic absorption spectrometry (AAS) system was used to quantify element concentrations based on the absorption of specific light wavelengths. The high concentrations of Mg and Zn were measured by flame AA and the trace elements (Cu, Mn, Co, and Fe) were determined by graphite furnace AA (GFAA). Full characterization of product monoclonal antibodies (mAbs) was undertaken by LCMS, MALDI and UHPLC. Peptide mapping, peptide sequencing, glycosylation, and oxidation of target mAbs analyzed by QTOF, triple quadrupole, and MALDI mass spectrometry was performed to characterize and control biologics production. Additionally, n-glycans were analyzed using UHPLC with fluorescence detection after labeling with pyridylamino (PA)-glycan and 2-aminobenzamide (2-AB)- glycan.
Chromatographic and Mass Spectrometry Approaches to Supporting Biologics Development
Monoclonal antibodies (mAbs) are a class of biotherapeutics that are rapidly gaining traction. The higher-order structures of mAbs play a crucial role in determining their efficacy and safety. It is crucial that mAbs are accurately characterized before they can become biotherapeutics. A better understanding of mAbs helps craft better bioprocesses, formulas, and dosages. In this study, a recombinant human IgG NIST mAb reference standard was analyzed using high resolution quadrupole time of flight liquid chromatography-mass spectrometry (QTOF-LCMS). An innovative proteolysis method called nano-surface and molecular-orientation limited proteolysis (nSMOL) selectively cleaves Fab regions of mAbs. Regardless of the type of antibodies scientists use, nSMOL can be used to develop analytical methodologies. Triple quadrupole liquid chromatography-mass spectrometry (TQ-LCMS) permits multiple reaction monitoring (MRM) to determine the level of fragmentation in peptides derived from Fab. Furthermore, a protocol for selecting pharmacokinetically suitable signature peptides was developed using nSMOL and TQ-LCMS. Additionally, consistency of glycosylation during process validation is a critical quality attribute (CQA) as N-linked glycans can impact the stability, solubility, and recognition of glycoproteins by glycan-binding proteins. Proteins are attached to N-glycans by covalent bonds formed by N-glycosidic bonds forming at the asparagine (Asn) residues. Monoclonal antibodies and biosimilars can cause such an event at the crystallizable fragment (Fc). In this study, we measured the released glycans from mAbs using QTOF-LCMS. A variety of methodologies are available for the characterization of mAbs and related biologics. Any kind of analysis can be performed based on our tailored workflows, including whole protein analysis, subunit, and peptide fragment analysis, and released N-Glycan analysis.
Featured LC-MS Applications
Monoclonal Antibody Workflows on the Shimadzu Q-TOF LCMS-9030 Using the Protein Metrics Software Suite
Accurate characterization of monoclonal antibodies is essential to development of biotherapeutics. Thorough understanding of biotherapeutic properties aids in the optimization of bioprocess production, product formulation, and product dosage. In this application note, we use the new Shimadzu Q-TOF LCMS-9030 to characterize the recombinant human IgG1ҝ NIST mAb reference standard as a model of biotherapeutic monoclonal antibodies.
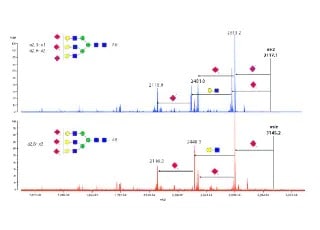
N-Linked Glycan Analysis of Monoclonal Antibodies, Biosimilars, and Glycoproteins with High Resolution Mass Spectrometry and Fluorescence Detection
The Shimadzu LCMS-9030 has shown excellent mass accuracy for N-linked glycans on several proteins. The Restek Raptor Polar X, an innovative hybrid ion-exchange/HILIC column, is an ideal column for glycans as reflected by the baseline separation of the structurally similar N-glycans. Protein Metrics Glycan Workflow offers additional workflows to other published methods.
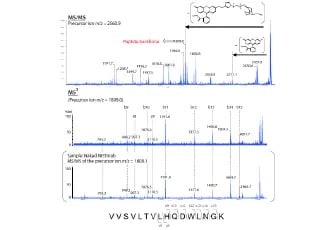
In-depth Peptide Mapping of Monoclonal Antibody (mAb) by A de novo Peptide Sequencing Method on Q-TOF Mass Spectrometer with Data-Independent Acquistion
In this report, we demonstrated an integrated MS full-scan and MS/MS Diag approach on Shimadzu LCMS-9030 (Q-TOF) mass spectrometer for de novo peptide sequencing of mAb.
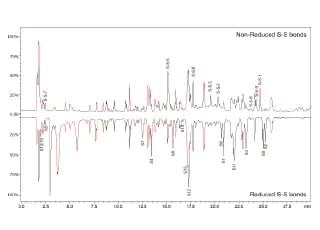
Disulfide Bond Characterization of Monoclonal Antibody (mAb) using Q-TOF Mass Spectrometer
A straightforward LCMS-based method for accurate disulfide bond peptide characterization of mAb biosimilar was established on LCMS-9030 (Q-TOF).The MS/MS spectra with fragmentation data provide high confidence results on sequencing analysis. The demonstrated performance for bevacizumab biosimilar in detection and de novo sequencing of non-reduced and reduced disulfide bonds signifies its practicability for the structural characterization of mAb biosimilars.
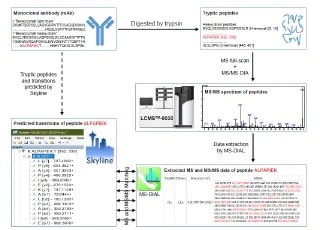
LCMS Bioanalysis of Antibody Drugs Using Fab-selective Proteolysis "nSMOL Method"
nSMOL (nano-surface and molecular-orientation limited proteolysis) is Shimadzu's completely new, proprietary, and innovative technique for selective proteolysis of Fab region of monoclonal antibodies. nSMOL allows analytical method development of antibody drugs independent of a variety of antibody drugs. Fab-derived peptide fragments produced by nSMOL can be precisely quantified by multiple reaction monitoring (MRM) using the Shimadzu LCMS-8050/8060 triple quadrupole liquid chromatograph mass spectrometer (TQ-LCMS). This report describes a selection protocol of signature peptides suitable for pharmacokinetic studies.
Featured MALDI Applications
Simplified Mass Measurement of Chemically-Modified Antibodies: Determination of the Presence of the Number of Modifications Using a Linear Benchtop MALDI-TOF MS
Antibody drug conjugates (ADC), a type of pharmaceutical composed of an antibody bound to a drug, appeared in the 2000s with the expectation they would serve as more effective anti-cancer drugs than previous small-molecule pharmaceuticals through the combination of the antibody's high selectivity and the availability of a small molecule drug. With a small number of products already available in the marketplace, the degree to which and where binding occurs are important characteristics to determine quality when compounds are artificially bound to proteins, as in the case of ADCs. This article introduces an example of analyzing the pseudo ADC, which was created by artificially binding low molecular compounds to a standard research antibody, using a benchtop MALDI-TOF MS.
Affinity purification of IdeZ digest for Glycosylation profile of Immunoglobulins using a linear benchtop MALDI-TOFMS.
Glycosylation on protein plays wide-range vital roles in biological processes from stabilization of protein conformation to expression of binding specificity. In this view, a characterization of the N-/O-linked glycan is quite significant, especially, in development of biopharmaceuticals. To date, whereas intensive efforts were conducted to characterize glycans precisely with high-end mass spectrometers, conventional instruments without time consuming preparation has been anticipated for batch analysis in screening or QA/QC. A newly developed bench-top MALDI-TOFMS is expected to be the conventional instrument in terms of sufficient specification, through-put, and cost effectiveness. We attempted to characterize glycosylation of IgG without a release of glycan using the bench-top MALDI-TOFMS. To do this, we examined a preparation using affinity beads and enzymatic cleavage.
Glycosylation Profile of IgGs Using a Linear Benchtop MALDI-TOFMS and Affinity Purification of Fc
Purification of enzymatically fragmented IgGs by affinity-beads enables batch analysis for the glycosylation using a bench-top MALDI-TOFMS, MALDI-8020. The MS resolution of MALDI-8020 is sufficient to recognize three Fc that mainly differ in glycosylation. A statistical analysis by eMSTAT Solution enables a classification of three glycosylated Fc smoothly and quickly, which could be applicable to QA/QC.
Analysis of N-Linked Glycan using MALDI-Mini-1 Compact MALDI Digital Ion Trap Mass Spectrometer: Structural Analysis and Identification of Sialyl Linkage Isomers
Focusing on a blood serum-derived N-linked glycan, this article introduces an example in which sialic acid residue was stabilized by using the sialic acid linkage specific alkylamidation (SALSA) method and detection and analysis were performed with a Shimadzu MALDImini-1 compact MALDI digital ion trap (MALDI-DIT) mass spectrometer.
Analysis of Modification Site of Chemically Modified Antibody Using MALDI-Mini-1 Compact MALDI DIT Mass Spectrometer
Antibody drug conjugates (ADC) are a new class of anti-cancer drugs in which an antibody is bound to a cytotoxic drug. Because they combine the high substrate specificity of the antibody and the effect of a low-molecular drug, ADC are expected to be more effective anti-cancer drugs than the conventional low-molecular drugs. When a different compound is bound artificially to a protein, the binding degree of that compound and its binding site become one of the critical quality properties. A pseudo ADC was created by artificially binding a low-molecular compound to a standard research antibody, and was then analyzed using a MALDImini-1.
Additional Applications
Analyses of Antibody Drugs Using Ultra High Performance Liquid Chromatography
This article introduces analyses of mAbs and ADCs for quality control using an inert UHPLC system “Nexera XS inert” that is extremely resistant to mobile phases containing high salt concentrations. The type of impurities and their amount are different depending on the type of antibody. There fore, it is note worthy that analytical conditions should be optimized for each sample. Thus, the optimization techniques will also be described in this article.
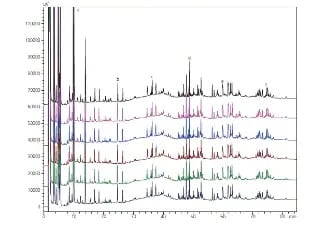
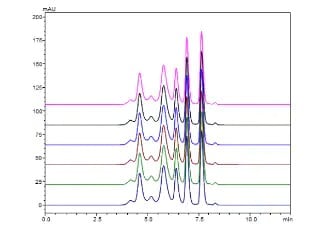
Analysis of protein drugs aggregation Using Size Exclusion Chromatography
This article describes an aggregate analysis using a Shimadzu “Shim-pack™ BioDiol”, size exclusion chromatography column with “Nexera XS inert”, an ultra high performance liquid chromatograph. This chromatograph has high salt tolerance and metal-free flow path and allows to use highly salted mobile phases and prevents sample adsorption.
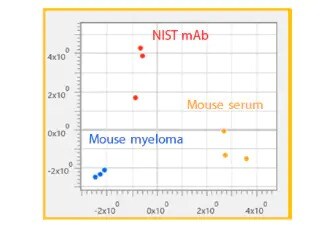
Method Optimization for the Analysis of Monoclonal Antibodies by Size-Exclusion Chromatography
Antibody drugs using monoclonal antibodies pose concerns over aggregates formed during production and storage and their impact on safety and efficacy. During monoclonal antibody production, aggregates formation is monitored, and size-exclusion chromatography is one of the most widely used techniques. However, size-exclusion chromatography is performed at relatively low flow rates and requires long analysis times. Analyses of monoclonal antibody drugs must also take into account interaction between the monoclonal antibodies and column packing materials. This Technical Report provides an example of using a column packed with small particle material to optimize an analytical method for analysis of monoclonal antibody aggregates. This article investigates the effect of mobile phase salt concentration, flow rate, and pH on chromatographic separation and peak shape. Moreover we describe an example of method optimization by using a dedicated software for improving separation, sensitivity and reducing analytical time.
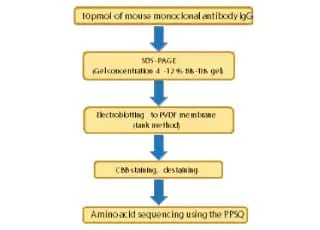
N-Terminal Amino Acid Sequencing of IgG Antibodies
This article introduces an example of amino acid sequencing of mouse antibody IgG using the PPSQ-51A/53A Protein Sequencer isocratic system as an instance of N-terminal amino acid sequencing of biomedicines.
Peptide Mapping of Antibody Drugs by Nexera-i
Peptide mapping by HPLC is one of the important quality assurance tests used for verifying the primary structure of antibody drugs. Typically, following enzymatic digestion of the antibodies, separation is conducted using a traditional reversed phase column. Due to the large number of peaks that require separation, the use of small-particle columns and core shell columns for peptide analysis has spread in recent years. In order to compare elution profiles for identity and mutation confirmation, a highly repeatable system is required. Here, the Nexera-i is used in the analysis of IgG (human immunoglobulin G) tryptic digest.
Optimizing Aggregate Analysis - Pore Size
In SEC ,smaller molecules enter the pores of the particle and proceed slowly along the axis direction of the column while molecules larger than the pore size are excluded from pores and elute at first from the column. In this application, we demonstrate the effect of pore size in SEC analysis.
Optimizing Aggregate Analysis - Mobile Phase
Despite being a simple assay, mobile phase condition is often needed to be optimized to improve peak shape and resolution of proteins. In this study, we describe analysis of trastuzumab, an anti-HER2 mAb using Shim-pack Bio Diol column on Nexera Bio UHPLC. The effect of mobile phase composition (ionic strength and pH) on chromatographic separation of trastuzumab is presented in this application news.
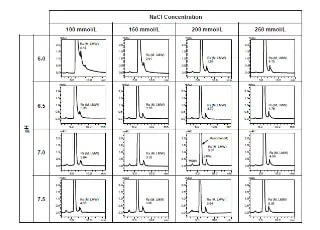
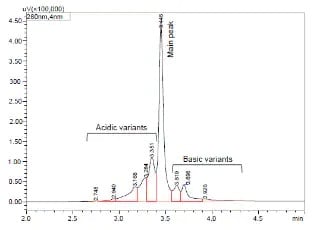
Charge Variant analysis of mAb Biosimilars by Nexera UHPLC with a Shim-pack Bio IEX Column
In this study, we describe both salt-gradient and pH-gradient methods for separating the charge variants of bevacizumab biosimilar using Shimadzu Nexera UHPLC and a Shim-pack Bio IEX column (4.6 mm x100 mm, 3 μm).
Exploring Factors that Influence Charge Variant Analysis of mAbs with Shim-pack Bio IEX Columns using Salt Gradient Elution
In this study, we describe a charge variant analysis of Trastuzumab using salt gradient with Shimadzu strong cation-exchange column, Shim-pack Bio IEX SP-NP. The separation efficiency under different gradient slopes, gradient times and flow rates are discussed.
Determination of Protein Secondary Structures of Monoclonal Antibody using FTIR Spectroscopy
Monoclonal antibodies (mAbs) are a major class of biopharmaceuticals due to its wide application in medicine and biological science. The antibody’s biological activity can be attributed to its unique structural conformation [1]. Protein higher order structure (HOS) includes the secondary, tertiary and quaternary structures of protein, which comprise the three dimensional structures of protein [2]. Analysis of the HOS of mAbs is essential to ensure the quality and efficacy of the protein therapeutics product. Some analytical methods that have been used to characterize protein HOS include UV circular dichroism, nuclear magnetic resonance (NMR) and fourier transform infrared (FTIR) spectroscopy. FTIR spectroscopy is suitable to determine the relative amount of different secondary structure of proteins. This information can be obtained from the amide band I of protein in the IR spectrum, ranging from 1600 cm-1 to 1700 cm-1. Mathematical procedures such as band curve-fitting and second derivatives can be applied to resolve the overlapping amide I band components and quantify the secondary structure of proteins. In this application news, the secondary structure of mAbs is examined by using FTIR spectroscopy and band curve-fitting data analysis.
News / Events
-
AAPS Pharm Sci 360 2025
November 9-12
Henry B. Gonzalez Convention Center
San Antonio, TX -
Pharma Community Network Event
Shimadzu Scientific Instruments and ZefSci invite you and your analytical teams to a pharma community networking event to celebrate 150 years of science and innovation at The Foundry & Lux in South San Francisco. Our event will be built around innovation, collaboration, and connection.
-
ASMS (American Society for Mass Spectrometry) 2025
June 1-5
Baltimore Convention Center
Baltimore, MD -
Shimadzu Scientific Instruments Opens Boston Location of Its R&D Center Focus will be on promoting customer-oriented development to expand business in the pharmaceutical field
Shimadzu Scientific Instruments, Inc. (SSI, Columbia, Maryland, USA), a Shimadzu Group company, has opened a satellite lab in Boston, Massachusetts to be the base of its collaborative research and development activities on the East Coast. Established to conduct research and development more closely linked to customers, SSI's R&D Center consists of three bases, with the main facilities at its Maryland headquarters, a West Coast location, and this new space in Boston near the city center. The Boston lab was set up by partnering with Labshares, a shared laboratory service provider for life science companies.