Environmental Solutions
Shimadzu Ion Chromatography
Ion chromatography is a form of liquid chromatography used to separate ions in aqueous solutions. Typically, IC analyzes anions such as fluoride, chloride, nitrite, bromide, nitrate, phosphate and sulfate. Cations by IC was approved for wastewater reporting in the 2012 method update. Ion chromatography can detect other ions as well. Chromate, perchlorate, and organic acids are examples.
Because different ions can be detected, good separation is necessary to avoid overlapping peaks of analyte and contaminants. Detection using conductivity is common; however, UV can detect some anions, such as nitrite and nitrate. UV is useful in solutions containing high concentrations of chloride. In these solutions the chloride peak and nitrite peaks overlap with conductivity detectors. UV detects nitrite and nitrate, but not chloride. Electrical conductivity detection is based on detecting the conductivity of ions. Voltage is applied to a pair of plates, and if ions are present, a current flows. If there are ions in the mobile phase as it passes through the detector flow-cell, a signal is produced that is proportional to the ion concentration. Each ion also has an equivalent conductivity constant that indicates how easily it conducts current.
There are two type of conductivity detection. Non-suppressed conductivity and suppressed conductivity. Most EPA-approved methods use the suppressed conductivity method. Standard Methods 4110C is EPA approved for wastewater and uses non-suppressed conductivity.
The simplest form of ion chromatography is non-suppressed conductivity. In this method, the mobile phase is a non-UV absorbing weak organic acid. Detection limits are high enough that the method is useful for the higher concentrations of anions in wastewater. The detector is positioned immediately after the mobile phase emerges from the column. The conductivity detector detects ions in the mobile phase, and cations and anions in the sample. This technique enables you to do ion chromatography using a normal HPLC.
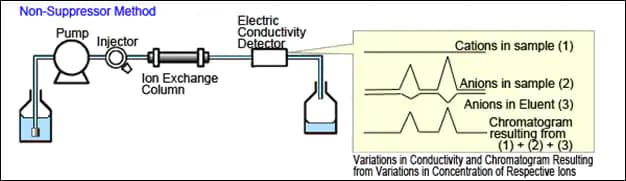
In non-suppressed conductivity, each ion passes through the column to the detector. As mentioned before, the technique is not that sensitive for anions, making it useful for wastewater. Cations' sensitivity is not significantly affected by suppressed or non-suppressed conductivity. Most IC manufactures will tell you "don't bother with non-suppressed conductivity methods". However, EPA has approved a non-suppressed method for anions in wastewater. If you are tired of diluting samples, just to put chloride and sulfate on scale, this may be an option.
The suppressor method (EPA Method 300.0 for example) changes the eluent composition to one with a lower electrical conductivity. Solutions with aqueous sodium carbonate or sodium hydroxide are used. The suppressor is attached between the column outlet and the detector. The suppressor exchanges all the cations for hydrogen ions. Because hydrogen ions have a higher equivalent electrical conductivity, the signal is increased. Thus, the sensitivity of suppressed conductivity is higher.
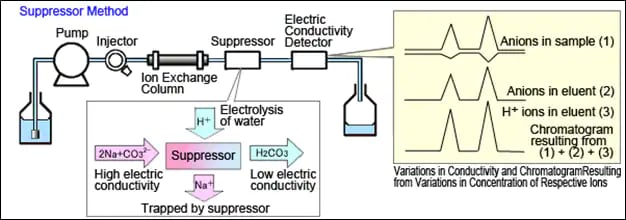
The suppressor serves to reduce background noise and increase response. These IC methods have lower detection limits for anions than non-suppressed methods.
Other Solutions
- Atomic Absorption
- Balances
- Energy Dispersive XRF
- Gas Chromatography
- GCMS
- Gas Chromatography Tandem Mass Spectrometry
- HPLC
- ICP/AES
- ICP/MS
- Ion Chromatography
- LCMS
- MALDI iD-Plus
- On-Line Analyzers
- Spectrophotometers
- Total Nitrogen Analyzers
- Total Organic Carbon Analyzers (TOC)
- Return to Environmental Top Page



