Environmental Solutions
Shimadzu Total Organic Carbon Analyzers (TOC)
Total organic carbon analyzers measure the amount of organic, inorganic, or total carbon in water or soil samples. TOC is an important indicator of disinfection byproducts and the byproduct rule requires drinking water facilities to measure TOC removal. TOC also correlates with BOD or COD in many matrices and can be used as a surrogate for those tests.
Analyze TOC by removing all the inorganic carbon and measuring NPOC, or calculate by subtracting inorganic carbon from total carbon. The subtraction method is prone to error and most people actually just measure NPOC.
The primary differences between TOC analyzers is the oxidation technique. Most TOC analyzers convert all carbon to carbon-dioxide and measure the CO2 with a CO2 specific NDIR detector. NDIR detection requires CO2 be swept by a carrier gas through the detector. It is more specific than membrane conductivity. Membrane conductivity diffuses CO2 through a membrane into an absorber solution. Any change in conductivity of the absorber solution measures CO2.
EPA-approved methods for TOC oxidize organic compounds using either high-temperature combustion (SM 5310B or ASTM D7573) or a wet chemical oxidation (SM 5310C or ASTM D4839). High-temperature combustion may occur by collision of gaseous phase sample with oxygen or by collision with oxygen on the surface of a catalyst. The catalyst-assisted oxidation allows lower temperatures to be used, increasing combustion tube life. Wet chemistry oxidation uses the persulfate ion. Persulfate oxidations are catalyzed by heat, UV irradiation, or a combination of the two.

Catalytic Combustion oxidizes organic compounds to CO2 and H2O at lower temperatures
The carrier gas containing oxygen passes over a hot metal catalyst. The oxygen molecules split to oxygen atoms that coat the surface of the catalyst. Injected sample, or fuel, splits into atoms on the surface of the catalyst. In Figure 1, the fuel is methane. A carbon from the methane collides with an oxygen atom to form carbon monoxide. Because of its affinity for the catalyst the CO molecule stays put until it collides with another oxygen to form CO2. The CO2 flies off into the carrier gas. The hydrogens from the methane also collide with oxygen to form OH- first and then H2O, which also enters the carrier gas. This process continues as the fuel percolates through the catalyst bed.
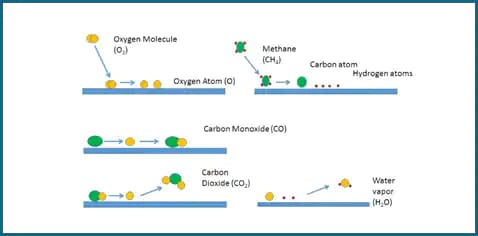
Once there is no more fuel, the catalyst recoats with oxygen from the carrier gas.
The mode, or mechanism, of collision is different with catalytic oxidation as compared to combustion. In combustion, the collisions occur in the gaseous phase, requiring very high temperatures to induce enough collisions in the shortest time possible. The carrier gas needs to be pure oxygen to ensure adequate collisions. Because the catalytic oxidation takes place on the surface of the catalyst, the temperature is lower. The lower temperature is below the melting point of sodium chloride, decreasing devitrification and increasing the life of the combustion tube.
Wet Chemical Oxidation TOC
Chemical oxidation reacts an oxidizing chemical with organic compounds to CO2 and H2O.
Under acid conditions, persulfate converts into a sulfate radical, which is a very strong oxidizer. Unlike catalytic combustion, which takes place between a gaseous and solid surface, persulfate reactions take place in solution. Persulfate is not a good oxidizer on its own, but its oxidizing power increases with heat or upon exposure to UV irradiation. The number of collisions between the oxidizer and the sample increases with temperature; unfortunately, the persulfate rapidly decomposes at high heat. In addition, other constituents of the sample matrix, particularly high chloride, react with persulfate, preventing it from oxidizing the carbon.
| Technique | Advantages | Disadvantages |
|---|---|---|
|
|
Lower temperature than HTC Tolerates High Salt |
Memory effects of catalyst Catalyst poisoning |
|
|
No memory effects |
Lower oxidation efficiency |
Choosing a TOC analyzer
Catalytic oxidation TOC analyzers operate at low combustion temperature of 680 ºC, making it possible to tolerate high salt content. Detection limits are higher than persulfate analyzers because you cannot inject as much sample. Persulfate analyzers have lower detection limits, but are more susceptible to interferences from chloride. Generally, for quantitation below 500 ppb in low chloride solutions get a persulfate analyzer. Above 2 ppm and for highly contaminated matrices go with catalytic combustion. Why? Catalytic combustion is faster, uses fewer reagents, and has a better oxidation efficiency (Table 1).
Other Solutions
- Atomic Absorption
- Balances
- Energy Dispersive XRF
- Gas Chromatography
- GCMS
- Gas Chromatography Tandem Mass Spectrometry
- HPLC
- ICP/AES
- ICP/MS
- Ion Chromatography
- LCMS
- MALDI iD-Plus
- On-Line Analyzers
- Spectrophotometers
- Total Nitrogen Analyzers
- Total Organic Carbon Analyzers (TOC)
- Return to Environmental Top Page


